Label: BIOVITA- bacillus subtilis, thiamine mononitrate, riboflavin, ascorbic acid, niacinamide, calcium phosphate, dibasic, dihydrate granule
-
Contains inactivated NDC Code(s)
NDC Code(s): 72689-0032-1 - Packager: OASIS TRADING
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 14, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
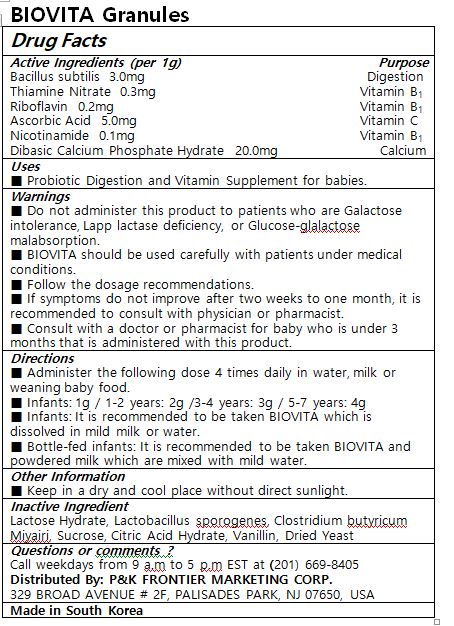
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
Administer the following dose 4 times daily in water, milk or weaning baby food.
Infants: 1g / 1-2 years: 2g /3-4 years: 3g / 5-7 years: 4g
Infants: It is recommended to be taken BIOVITA which is dissolved in mild milk or water.
Bottle-fed infants: It is recommended to be taken BIOVITA and powdered milk which are mixed with mild water. -
WARNINGS
Do not administer this product to patients who are Galactose intolerance, Lapp lactase deficiency, or Glucose-glalactose malabsorption.
BIOVITA should be used carefully with patients under medical conditions.
Follow the dosage recommendations.
If symptoms do not improve after two weeks to one month, it is recommended to consult with physician or pharmacist.
Consult with a doctor or pharmacist for baby who is under 3 months that is administered with this product.
Keep in a dry and cool place without direct sunlight.
- KEEP OUT OF REACH OF CHILDREN
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BIOVITA
bacillus subtilis, thiamine mononitrate, riboflavin, ascorbic acid, niacinamide, calcium phosphate, dibasic, dihydrate granuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72689-0032 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACILLUS SUBTILIS (UNII: 8CF93KW41W) (BACILLUS SUBTILIS - UNII:8CF93KW41W) BACILLUS SUBTILIS 3 mg in 1 g CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) (ANHYDROUS DIBASIC CALCIUM PHOSPHATE - UNII:L11K75P92J) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE 20 mg in 1 g THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 0.3 mg in 1 g RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 0.2 mg in 1 g ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 5 mg in 1 g NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 0.1 mg in 1 g Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SUCROSE (UNII: C151H8M554) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) VANILLIN (UNII: CHI530446X) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) BACILLUS COAGULANS (UNII: ISK1LOY57E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72689-0032-1 120 g in 1 JAR; Type 0: Not a Combination Product 12/25/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/25/2018 Labeler - OASIS TRADING (689991468) Registrant - OASIS TRADING (689991468) Establishment Name Address ID/FEI Business Operations ILDONG PHARMACEUTICAL CO., LTD. 689846555 manufacture(72689-0032) Establishment Name Address ID/FEI Business Operations OASIS TRADING 689991468 label(72689-0032)