DOK- docusate sodium capsule, liquid filled
Major Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Major 44-655 Delisted
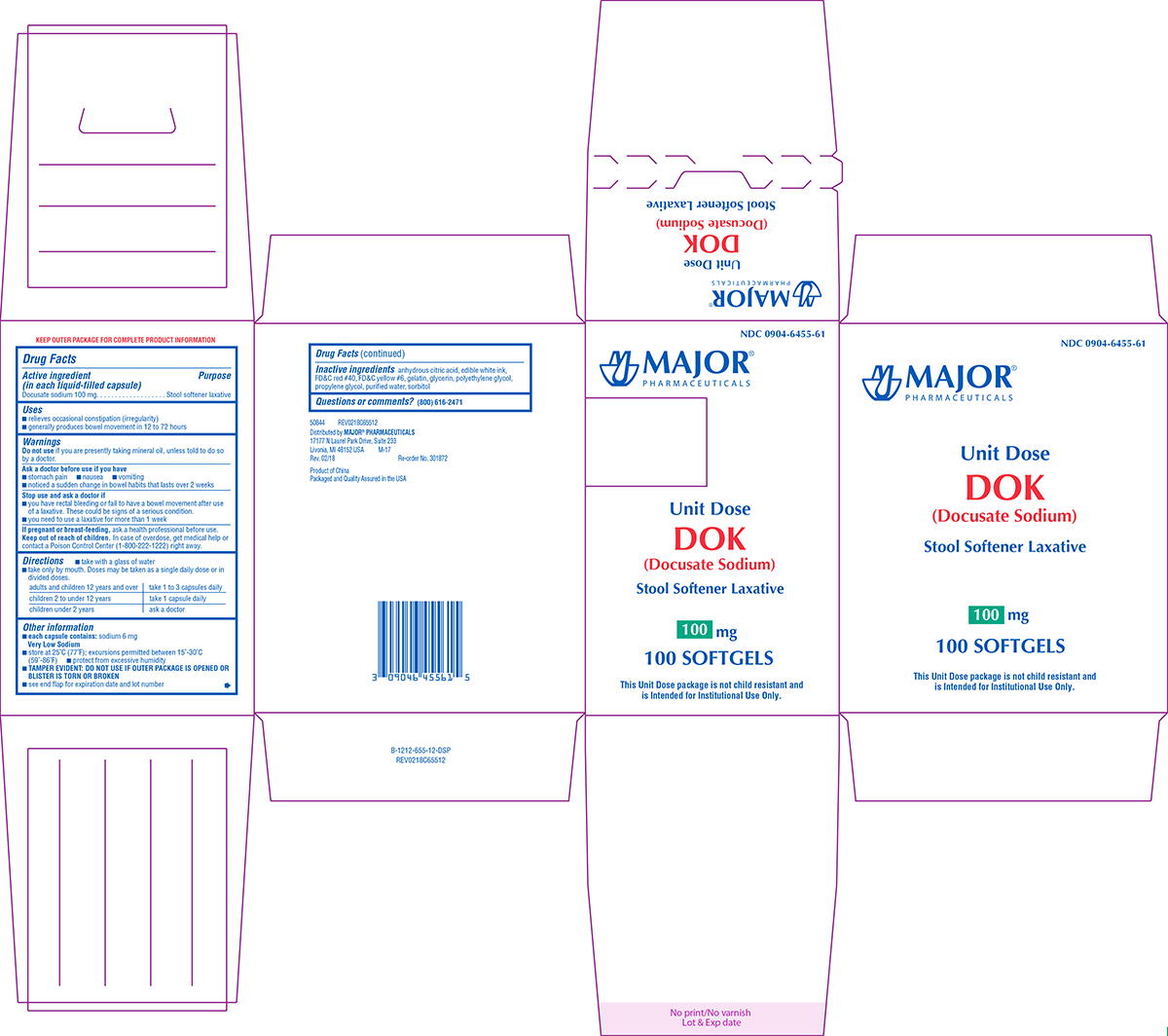
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 12 to 72 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Directions
- take with a glass of water
- take only by mouth. Doses may be taken as a single daily dose or in divided doses.
| adults and children 12 years and over | take 1 to 3 capsules daily |
| children 2 to under 12 years | take 1 capsule daily |
| children under 2 years | ask a doctor |
Other information
- each capsule contains: sodium 6 mg Very Low Sodium
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- protect from excessive humidity
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- see end flap for expiration date and lot number
Inactive ingredients
anhydrous citric acid, edible white ink, FD&C red #40, FD&C yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol, purified water, sorbitol
Principal Display Panel
NDC 0904-6455-61
MAJOR®
PHARMACEUTICALS
Unit Dose
DOK
(Docusate Sodium)
Stool Softener Laxative
100 mg
100 SOFTGELS
This Unit Dose package is not child resistant and
is Intended for Institutional Use Only.
50844 REV0218C65512
Distributed by MAJOR® PHARMACEUTICALS
17177 N Laurel Park Drive, Suite 233
Livonia, MI 48152 USA M-17
Rev. 02/18 Re-order No. 301872
Product of China
Packaged and Quality Assured in the USA
Major 44-655
| DOK
docusate sodium capsule, liquid filled |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(0904-6455) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | pack(0904-6455) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | pack(0904-6455) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(0904-6455) | |