NEUTROGENA AGELESS INTENSIVES ANTI WRINKLE DEEP WRINKLE DAILY MOISTURIZER SUNSCREEN BROAD SPECTRUM SPF20- avobenzone, homosalate, octisalate, octocrylene, and oxybenzone cream
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
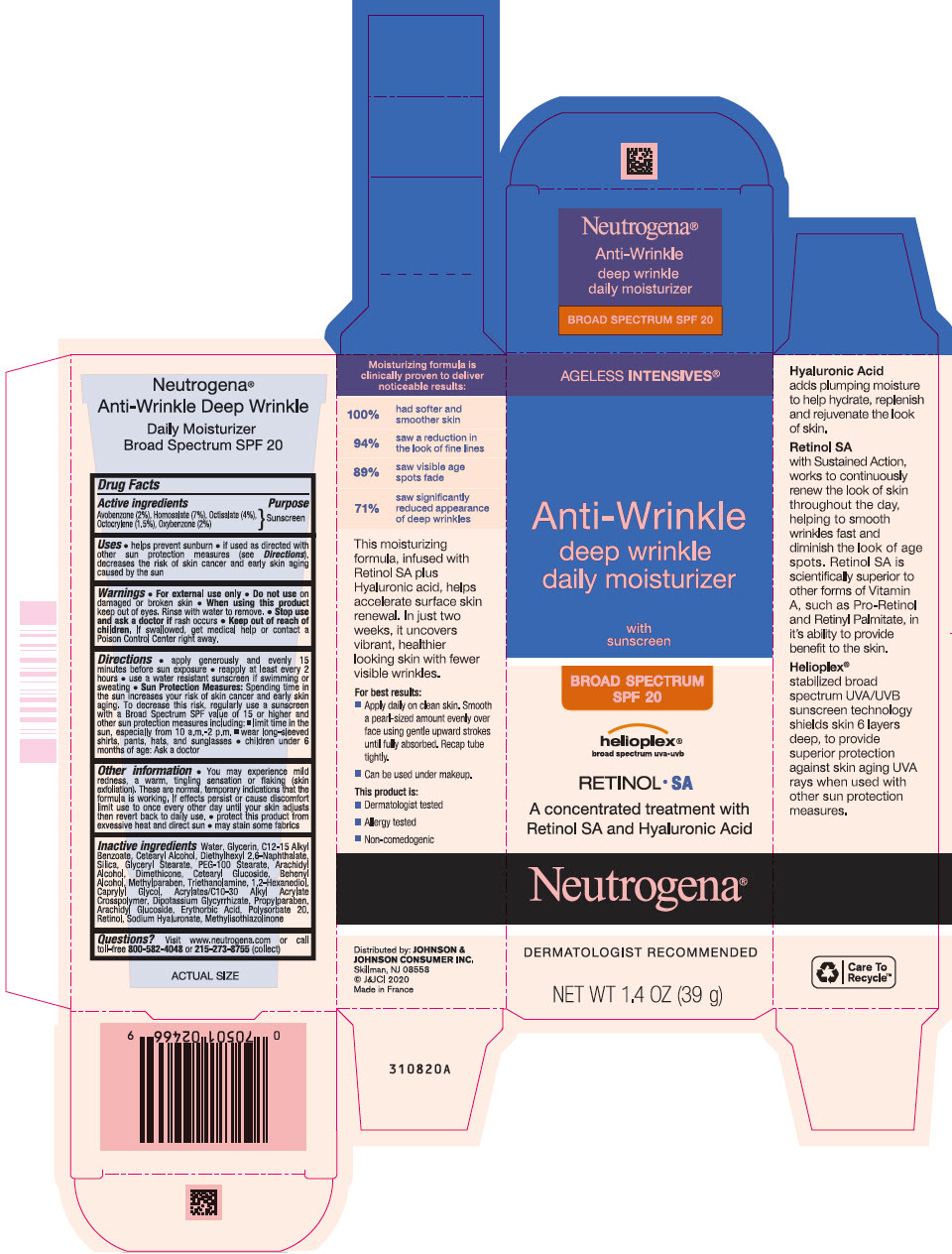
Neutrogena ® AGELESS INTENSIVES ® Anti-Wrinkle deep wrinkle daily moisturizer with sunscreen BROAD SPECTRUM SPF 20
Active ingredients
Avobenzone (2%)
Homosalate (7%)
Octisalate (4%)
Octocrylene (1.5%)
Oxybenzone (2%)
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- apply generously and evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor
Other Information
- You may experience mild redness, a warm, tingling sensation or flaking (skin exfoliation). These are normal, temporary indications that the formula is working. If effects persist or cause discomfort, limit use to once every other day until your skin adjusts, then revert back to daily use.
- protect this product from excessive heat and direct sun
- may stain some fabrics
Inactive ingredients
Water, Glycerin, C12-15 Alkyl Benzoate, Cetearyl Alcohol, Diethylhexyl 2,6 Naphthalate, Silica, Glyceryl Stearate, PEG-100 Stearate, Arachidyl Alcohol, Dimethicone, Cetearyl Glucoside, Behenyl Alcohol, Methylparaben, Triethanolamine, 1,2-Hexanediol, Caprylyl Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Dipotassium Glycyrrhizate, Propylparaben, Arachidyl Glucoside, Erythorbic Acid, Polysorbate 20, Retinol, Sodium Hyaluronate, Methylisothiazolinone
PRINCIPAL DISPLAY PANEL - 39 g Tube Carton
AGELESS INTENSIVES ®
Anti-Wrinkle
deep wrinkle
daily moisturizer
with
sunscreen
BROAD SPECTRUM
SPF 20
helioplex
®
broad spectrum uva • uvb
RETINOL • SA
A concentrated treatment with
Retinol SA and Hyaluronic Acid
Neutrogena
®
DERMATOLOGIST RECOMMENDED
NET WT 1.4 OZ (39g)
| NEUTROGENA AGELESS INTENSIVES ANTI WRINKLE DEEP WRINKLE DAILY MOISTURIZER
SUNSCREEN BROAD SPECTRUM SPF20
avobenzone, homosalate, octisalate, octocrylene, and oxybenzone cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (118772437) |