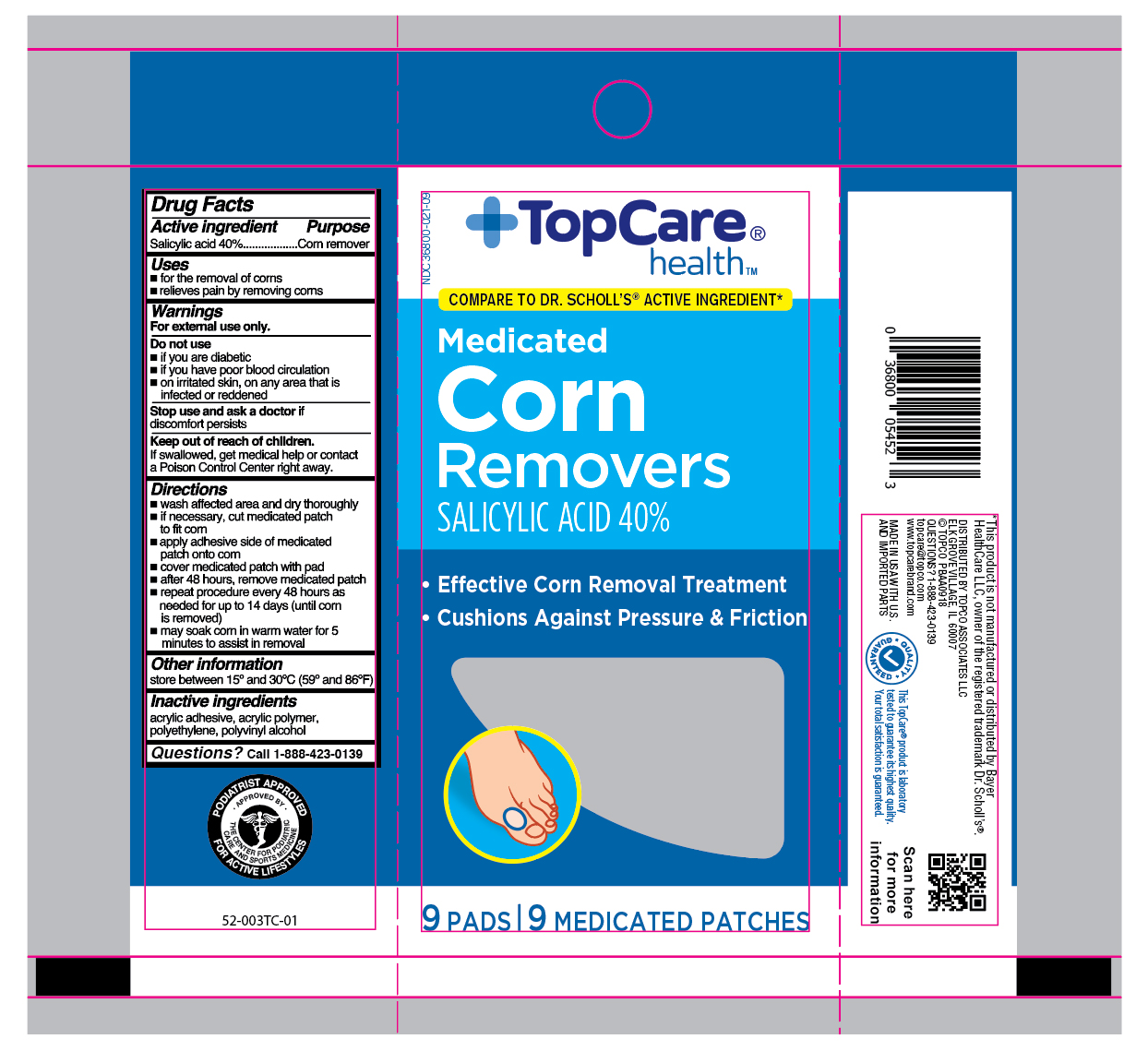
Label: SALICYLIC ACID- medicated corn removers patch
- NDC Code(s): 36800-021-09
- Packager: Topco Associates LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 8, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- wash affected area and dry area thoroughly
- if necessary, cut medicated patch to fit corn
- apply adhesive side down of medicated patch onto corn
- cover medicated patch with pad
- after 48 hours, remove medicated patch
- repeat procedure every 48 hours as needed for up to 14 days (until corn is removed)
- may soak corn in warm water for 5 minutes to assist in removal
- Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SALICYLIC ACID
medicated corn removers patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:36800-021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 40 mg in 9 Inactive Ingredients Ingredient Name Strength POLYVINYL ALCOHOL (UNII: 532B59J990) VINYL ACETATE (UNII: L9MK238N77) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:36800-021-09 9 in 1 PACKAGE; Type 0: Not a Combination Product 01/03/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M030 01/03/2012 Labeler - Topco Associates LLC (006935977)