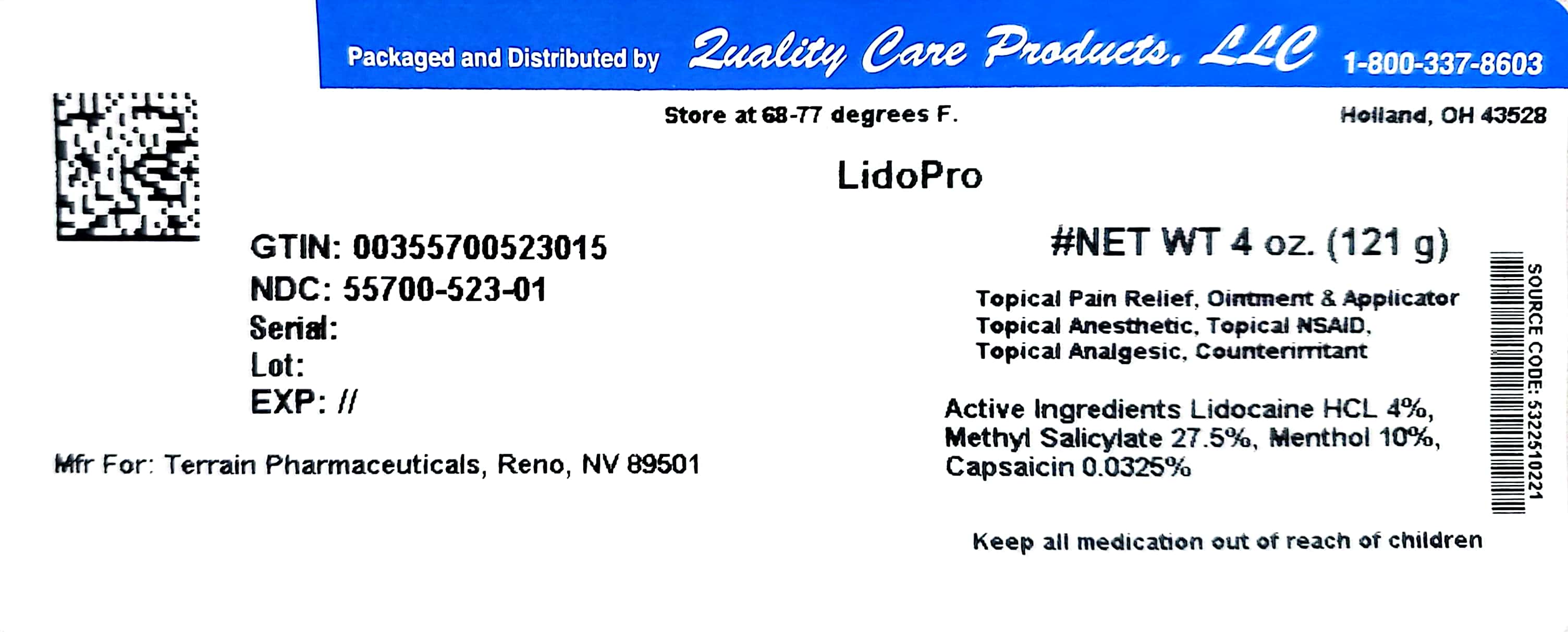
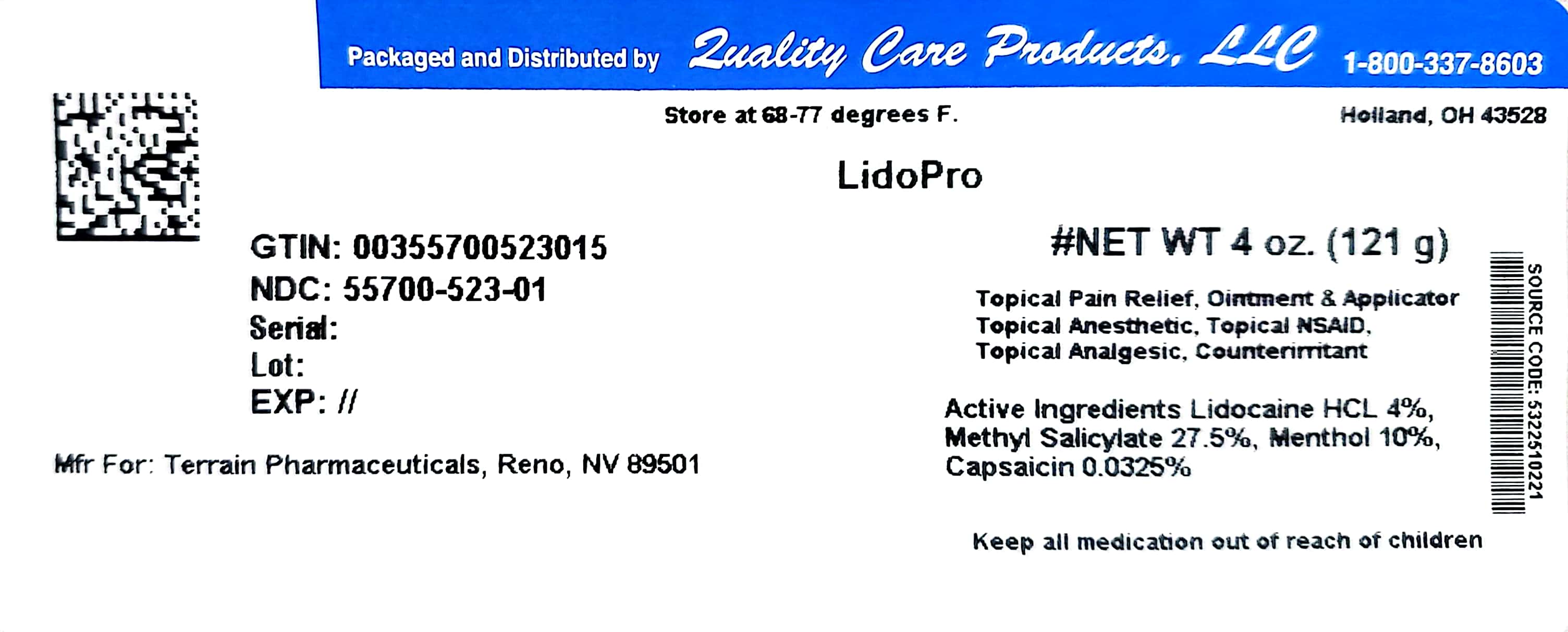
Label: LIDOPRO- capsaicin, lidocaine, menthol, and methyl salicylate ointment
- NDC Code(s): 55700-523-01
- Packager: Lake Erie Medical DBA Quality Care Products LLC
- This is a repackaged label.
- Source NDC Code(s): 53225-1022
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 21, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Uses:
-
Warnings
For external use only
Do not use
- on open wounds, cuts, damaged or infected skin
- with bandage or a heating pad
- if condition worsens or symptoms persists for more than 7 days
- excessive skin irritation occurs
When using this product
- avoid contact with eyes, genitals, and other mucus membranes. If eye contact occurs, rinse thoroughly with water.
- on open wounds, cuts, damaged or infected skin
- Directions
-
Inactive Ingredients
Allantoin, Aloe Barbadensis Leaf Juice, Ammonium Acryloyldimethyltaurate/VP Copolymer, Cetyl Alcohol, Chamomilla Recutita Matricaria Flower Extract, Dimethicone, Disodium EDTA, Ethylhexylglycerin, Glycerin, Glyceryl Stearate, Inulin Lauryl Carbamate, PEG-100 Stearate, Phenoxyethanol, Stearic Acid, Triethanolamine, Water.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIDOPRO
capsaicin, lidocaine, menthol, and methyl salicylate ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55700-523(NDC:53225-1022) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.000325 g in 1 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 0.04 g in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.1 g in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 0.275 g in 1 g Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) CETYL ALCOHOL (UNII: 936JST6JCN) CHAMOMILE (UNII: FGL3685T2X) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55700-523-01 121 g in 1 BOTTLE; Type 0: Not a Combination Product 05/19/2017 08/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/2014 08/31/2024 Labeler - Lake Erie Medical DBA Quality Care Products LLC (831276758) Establishment Name Address ID/FEI Business Operations Lake Erie Medical DBA Quality Care Products LLC 831276758 relabel(55700-523)