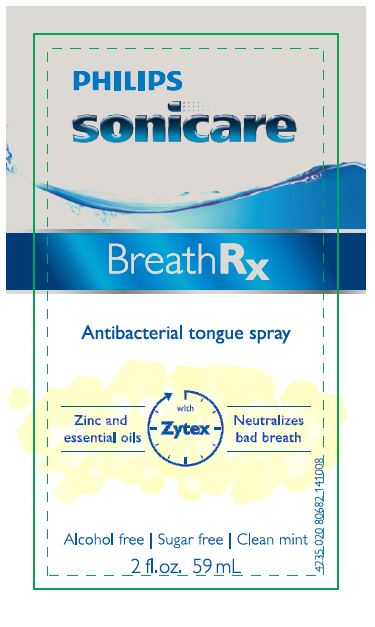
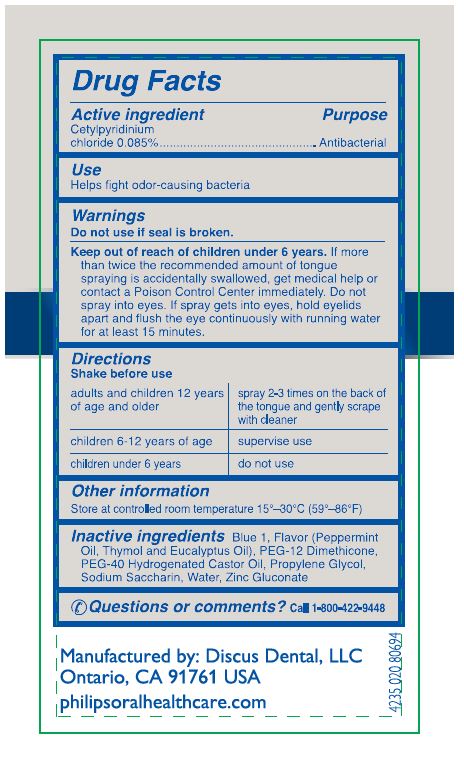
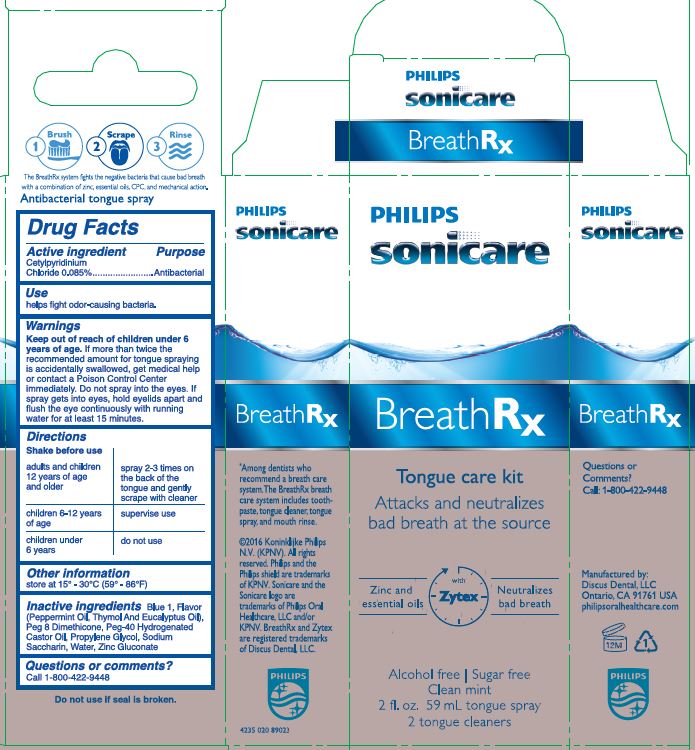
BREATHRX WITH ZYTEX- cetylpyridinium chloride spray
Discus Dental, LLC
----------
Warnings
Keep out of reach of children under 6 years of age. If more than twice the recommended amount for tongue spraying is accidentally swallowed, get medical help or contact a Poison Control Center immediately. Do not spray into the eyes. If spray gets into eyes, hold eyelids apart and flush the eye continuously with running water for at least 15 minutes.
| BREATHRX WITH ZYTEX
cetylpyridinium chloride spray |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Discus Dental, LLC (831726109) |
| Registrant - Discus Dental, LLC (831726109) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Discus Dental, LLC | 831726109 | manufacture(64854-012) | |
Revised: 1/2024
Document Id: 0f14a29a-6671-1640-e063-6294a90a539d
Set id: 7be46858-d6eb-45e3-a6c5-dbe394db04da
Version: 15
Effective Time: 20240116
Discus Dental, LLC