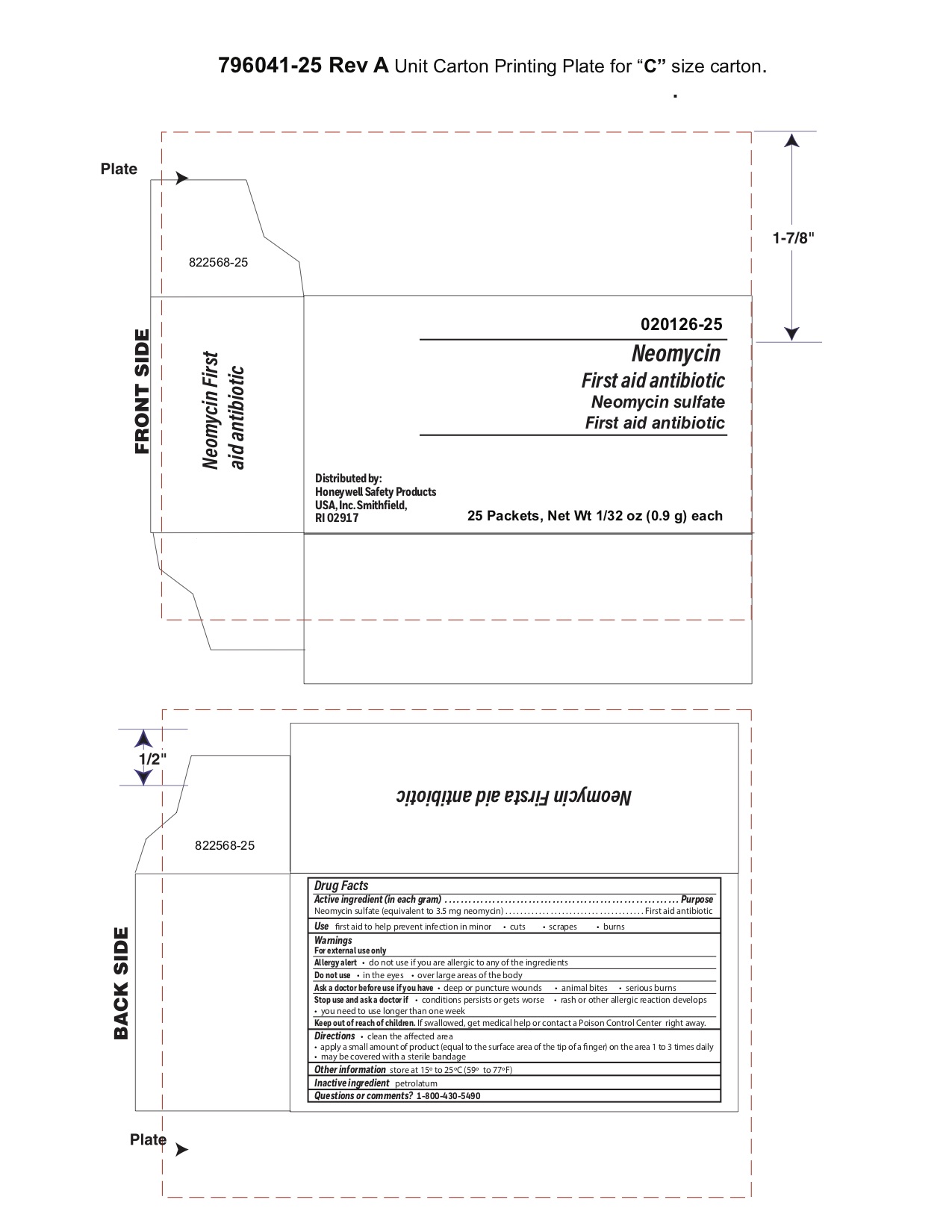
Neomycin
Active ingredient (each gram contains)
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Neomycin
Purpose
First aid antibiotic
Neomycin
Uses
first aid to help prevent infection in
Neomycin
Warnings
For external use only
Do not use
- in the eyes
- over large areas of the body
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- the condition persists or gets worse
- a rash or other allergic reaction develops
- you need to use longer than 1 week
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Neomycin
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Neomycin
Other information
- store at 15
o to 25
oC (59
o to 77
oF)
Neomycin
Inactive ingredient
petrolatum
Neomycin
Questions?
1-800-430-5490
BZK
Active ingredient
Benzalkonium chloride 0.13%
BZK
Purpose
First aid antiseptic
BZK
Uses
Antiseptic cleansing of face, hands, and body without soap and water
BZK
Warnings
For external use only
BZK
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
Stop use and ask a doctor if
- if irritation, redness or other symptoms develop
- the condition persists or gets worse
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
BZK
Directions
tear open packet and use as a washcloth
BZK
Other information
- store at room temperature 15
o to 30
oC (59
o to 86
oF)
- do not reuse towelette
BZK
Inactive ingredient
water
BZK
Questions
1-800-430-5490
Burn Cream
Active ingredient
Benzalkonium chloride 0.13%
Lidocaine HCl 0.5%
Burn Cream
Purpose
First aid antiseptic
External analgesic
Burn Cream
Uses
- prevent skin infection
- for tempoorary relief of pain associated with minor burns
Burn Cream
Warnings
For external use only
Burn Cream
Do not use
- in or near the eyes
- if you are allergic to any of the ingredients
- in large areas of the body particularly over raw surfaces or blistered areas
- for more than 10 days
Burn Cream
Ask a doctor beforef use if you have
- deep or puncture wounds
- animal bites
- serious burns
Burn Cream
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
Burn Cream
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Burn Cream
Directions
-
adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product (equal to the surface area of the tip of a finger) onto the affected area 1 to 3 times daily
- may be covered with a sterile bandage
-
children under 2 years of age: ask a doctor
Burn Cream
Other information
- tamper evident sealed packets
- do not use if packet is opened or torn
Burn Cream
Inactive ingredients
aloe barbadensis juice, cetyl alcohol, diazolidinyl urea, edetate disodium, glycerin, glyceryl stearate SE, methylparaben, mineral oil, PEG-100, propylene glycol, propylparaben, stearic acid, trolamine, water
Burn Cream
Questions or comments?
1-800-430-5490
Neomycin
Principal Display Panel
BZK
Principal Display Panel

Burn Cream
Principal Display Panel

4049 Kit Label
010101-4354L




