BREATHRX WITH ZYTEX- cetylpyridinium chloride rinse
Discus Dental, LLC
----------
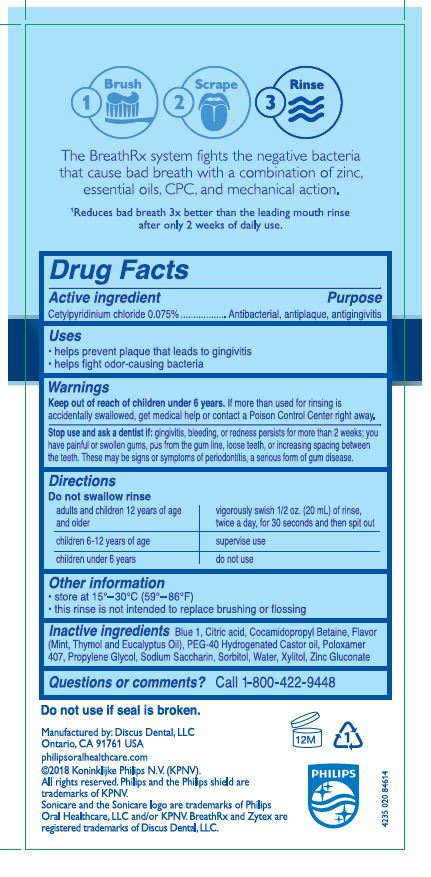
Warnings
If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Do not use if seal is broken
Directions
Do not swallow rinse. Adults and children 12 years of age and older: vigorously swish 1/2 oz. (20 mL) of rinse, twice a day, for 30 seconds then spit out. Children 6 years to under 12 years: supervise use. Children under 6 years of age: do not use
| BREATHRX WITH ZYTEX
cetylpyridinium chloride rinse |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Discus Dental, LLC (831726109) |
| Registrant - Discus Dental, LLC (831726109) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Discus Dental, LLC | 831726109 | manufacture(64854-014) | |
Revised: 1/2024
Document Id: 0f1506e8-986c-7c4c-e063-6394a90a2b31
Set id: 7bd694da-07bd-38e4-e053-2991aa0a6c56
Version: 15
Effective Time: 20240116
Discus Dental, LLC