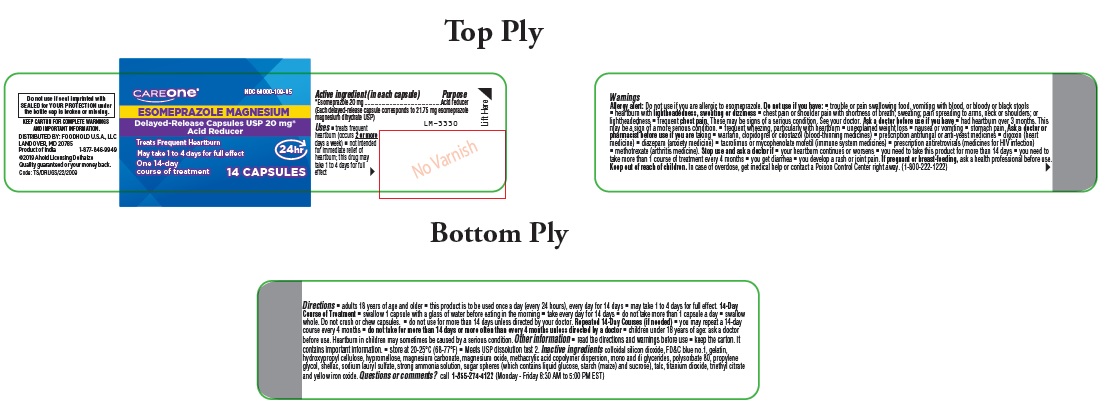
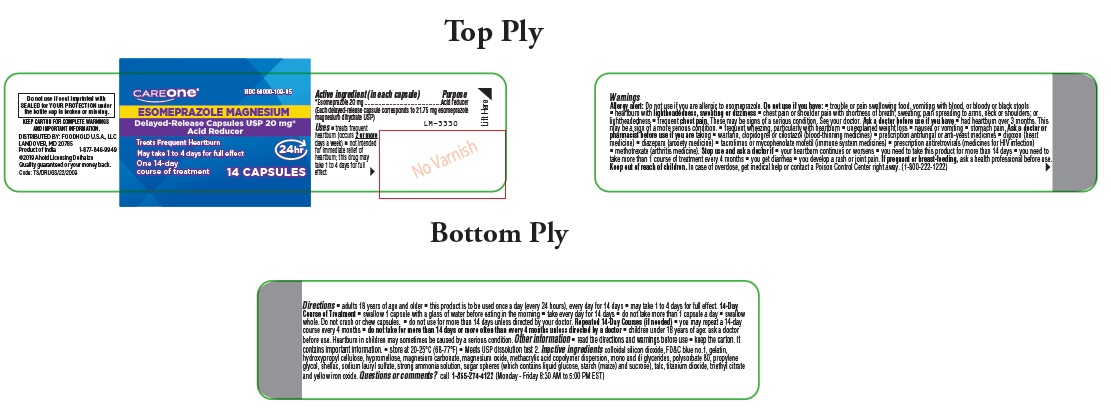
Label: ESOMEPRAZOLE MAGNESIUM capsule, delayed release
- NDC Code(s): 60000-109-03, 60000-109-05
- Packager: Ahold U.S.A., Inc,
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 15, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Uses
- Warnings
-
Do not use if you have:
- trouble or pain swallowing food, vomiting with blood, or bloody or black stools
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
These may be signs of a serious condition. See your doctor.
- Ask a doctor before use if you have
-
Ask a doctor or pharmacist before use if you are taking
- warfarin, clopidogrel or cilostazol (blood-thinning medicines)
- prescription antifungal or anti-yeast medicines
- digoxin (heart medicine)
- diazepam (anxiety medicine)
- tacrolimus or mycophenolate mofetil (immune system medicines)
- prescription antiretrovirals (medicines for HIV infection)
- methotrexate (arthritis medicine)
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- adults 18 years of age and older
- this product is to be used once a day (every 24 hours), every day for 14 days
- may take 1 to 4 days for full effect
14-Day Course of Treatment
- swallow 1 capsule with a glass of water before eating in the morning
- take every day for 14 days
- do not take more than 1 capsule a day
- swallow whole. Do not crush or chew capsules.
- do not use for more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
- you may repeat a 14-day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
Other information
- read the directions and warnings before use
- keep the carton. It contains important information.
- store at 20-25°C (68-77°F)
- Meets USP dissolution test 2
-
Inactive ingredients
colloidal silicon dioxide, FD&C blue no.1, gelatin, hydroxypropyl cellulose, hypromellose, magnesium carbonate, magnesium oxide, methacrylic acid copolymer dispersion, mono and di glycerides, polysorbate 80, propylene glycol, shellac, sodium lauryl sulfate, strong ammonia solution, sugar spheres (which contains liquid glucose, starch (maize) and sucrose), talc, titanium dioxide, triethyl citrate and yellow iron oxide.
Questions or comments?
call 1-855-274-4122 (Monday – Friday 8:30 AM to 5:00 PM EST)
DISTRIBUTED BY: FOODHOLD U.S.A., LLC
LANDOVER, MD 20785
Product of India
Code: TS/DRUGS/22/2009
1-877-846-9949
©2019 Ahold Licensing Delhaize
Quality guaranteed or your money back. - PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg (14 Capsule Container Label)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg (14 Capsule Container Carton)
-
INGREDIENTS AND APPEARANCE
ESOMEPRAZOLE MAGNESIUM
esomeprazole magnesium capsule, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60000-109 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESOMEPRAZOLE MAGNESIUM DIHYDRATE (UNII: 36H71644EQ) (ESOMEPRAZOLE - UNII:N3PA6559FT) ESOMEPRAZOLE 20 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM CARBONATE (UNII: 0E53J927NA) MAGNESIUM OXIDE (UNII: 3A3U0GI71G) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) AMMONIA (UNII: 5138Q19F1X) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 14mm Flavor Imprint Code I81 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60000-109-05 1 in 1 CARTON 10/16/2017 1 14 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:60000-109-03 3 in 1 CARTON 10/16/2017 2 14 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209339 10/16/2017 Labeler - Ahold U.S.A., Inc, (188910863) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650381903 ANALYSIS(60000-109) , MANUFACTURE(60000-109)