Label: ACNE CLEANSING- salicylic acid gel
- NDC Code(s): 49527-045-01
- Packager: CLINIQUE LABORATORIES LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
-
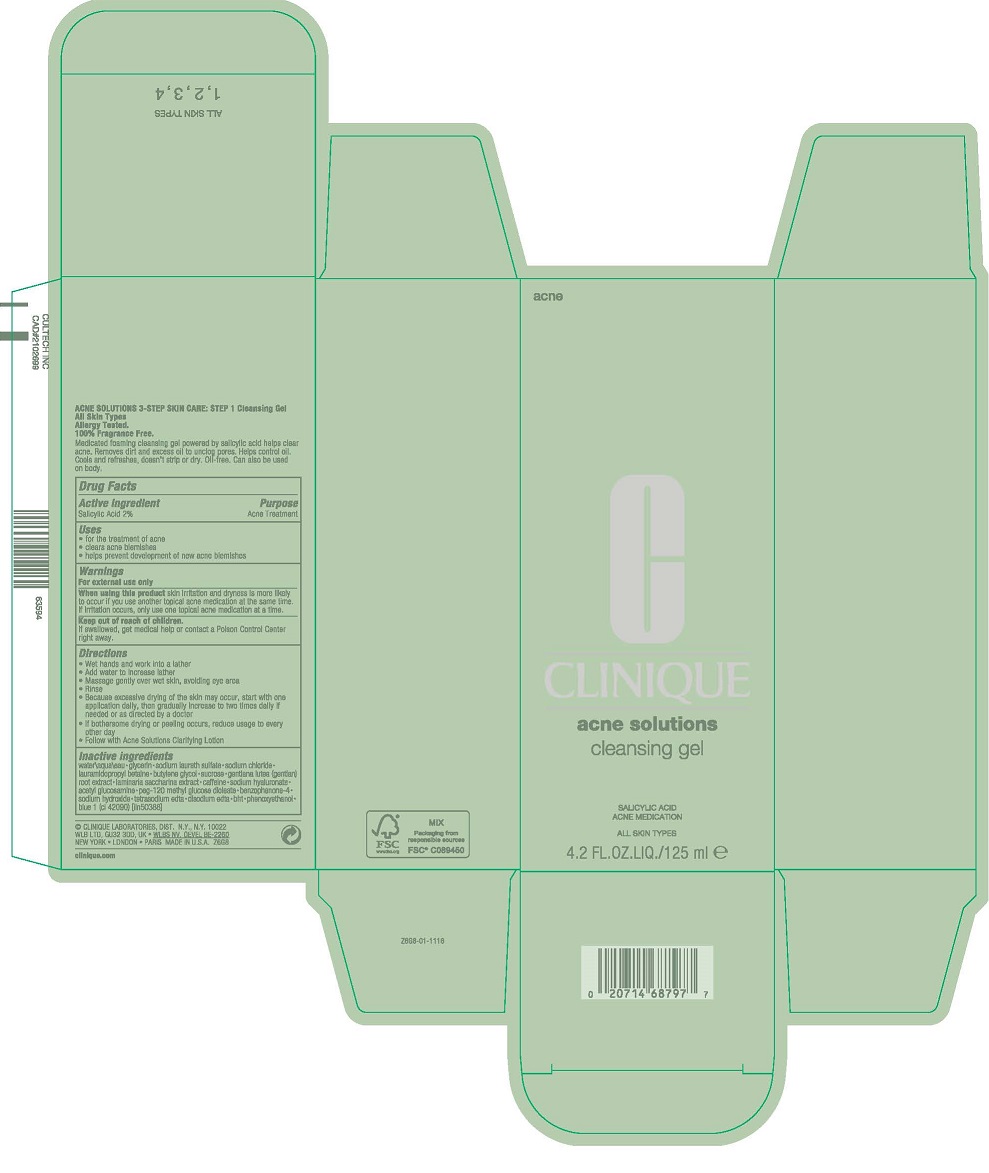
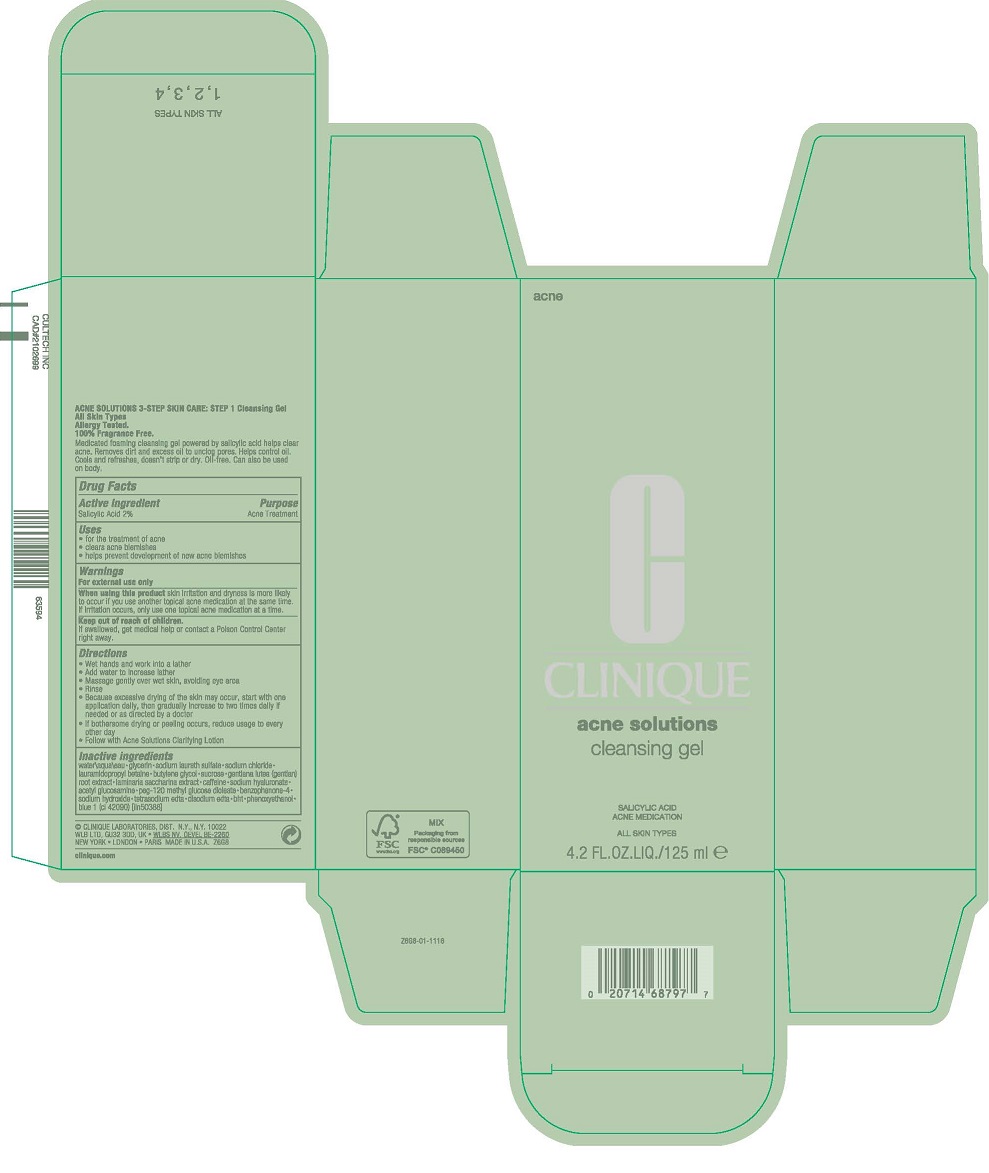
Inactive ingredients
water\aqua\eau [] glycerin [] sodium laureth sulfate [] sodium chloride [] lauramidopropyl betaine [] butylene glycol [] sucrose [] gentiana lutea (gentian) root extract [] laminaria saccharina extract [] caffeine [] sodium hyaluronate [] acetyl glucosamine [] peg-120 methyl glucose dioleate [] benzophenone-4 [] sodium hydroxide [] tetrasodium edta [] disodium edta [] bht [] phenoxyethanol [] blue 1 (ci 42090) <iln50388>
- Other information
- PRINCIPAL DISPLAY PANEL - 125 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
ACNE CLEANSING
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49527-045 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) SODIUM CHLORIDE (UNII: 451W47IQ8X) LAURAMIDOPROPYL BETAINE (UNII: 23D6XVI233) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SUCROSE (UNII: C151H8M554) GENTIANA LUTEA ROOT (UNII: S72O3284MS) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) CAFFEINE (UNII: 3G6A5W338E) HYALURONATE SODIUM (UNII: YSE9PPT4TH) N-ACETYLGLUCOSAMINE (UNII: V956696549) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) SULISOBENZONE (UNII: 1W6L629B4K) SODIUM HYDROXIDE (UNII: 55X04QC32I) EDETIC ACID (UNII: 9G34HU7RV0) EDETATE SODIUM (UNII: MP1J8420LU) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PHENOXYETHANOL (UNII: HIE492ZZ3T) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49527-045-01 1 in 1 CARTON 10/01/2014 1 125 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 10/01/2014 Labeler - CLINIQUE LABORATORIES LLC (044475127) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(49527-045) , pack(49527-045) , label(49527-045)