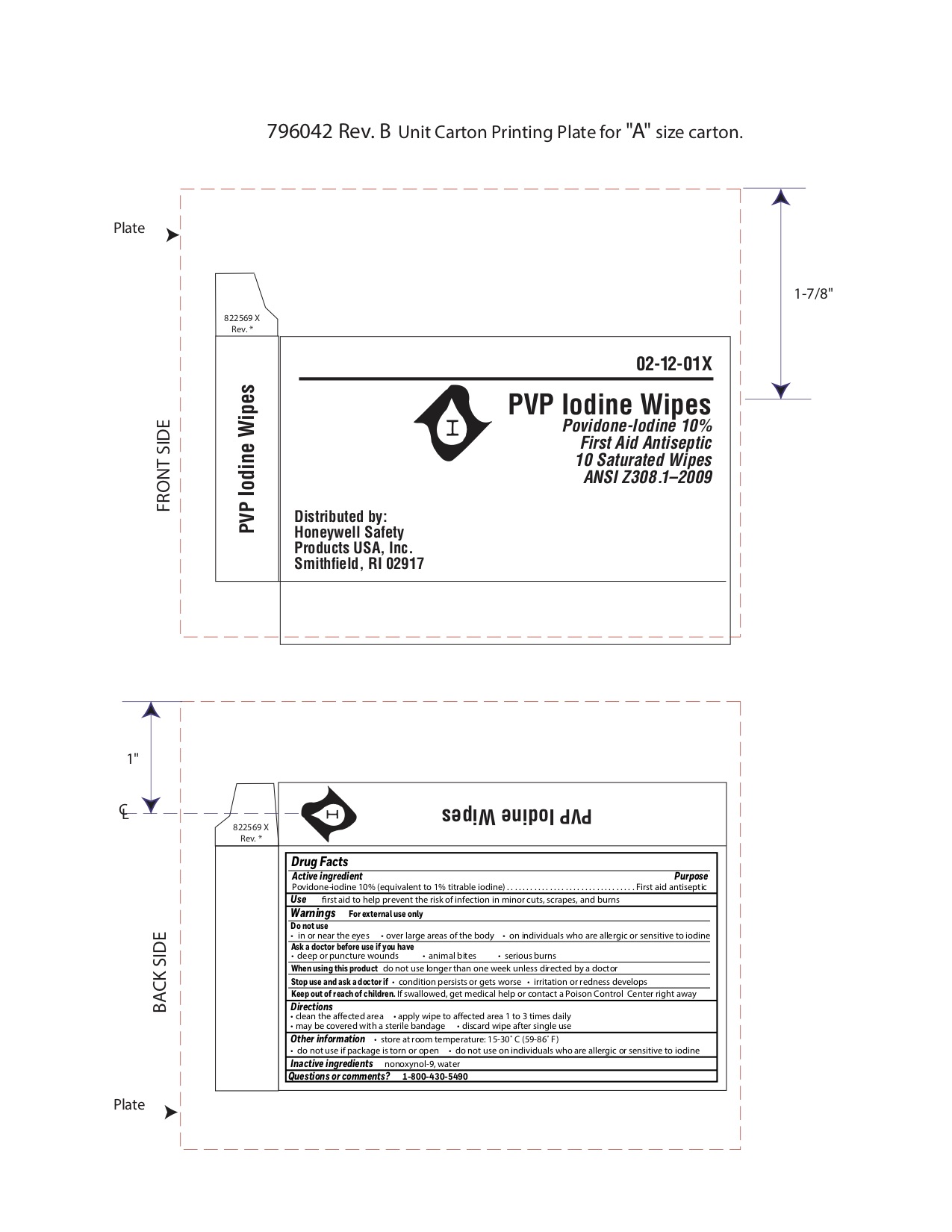
PVP Wipes
Active ingredient
Povidone-iodine 10%
(equivalent to 1% titratable iodine)
PVP Wipes
Purpose
First aid antiseptic
PVP Wipes
Uses
- first aid antiseptic to help prevent infection in minor cuts, scrapes and burns
PVP Wipes
Warnings
For external use only.
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition worsens or persists for more than 72 hours
- irritation and redness develops
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
PVP Wipes
Directions
- clean the affected area
- apply1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
- discard wipe after single use
PVP Wipes
Other information
- do not use on individuals who are allergic or sensitive to iodine
- store at controlled temperature 59-86ºF (15-30ºC)
- do not use if pouch is open or torn
PVP Wipes
Inactive ingredients
nonoxynol 9, water
PVP Wipes
Questions
800-430-5490
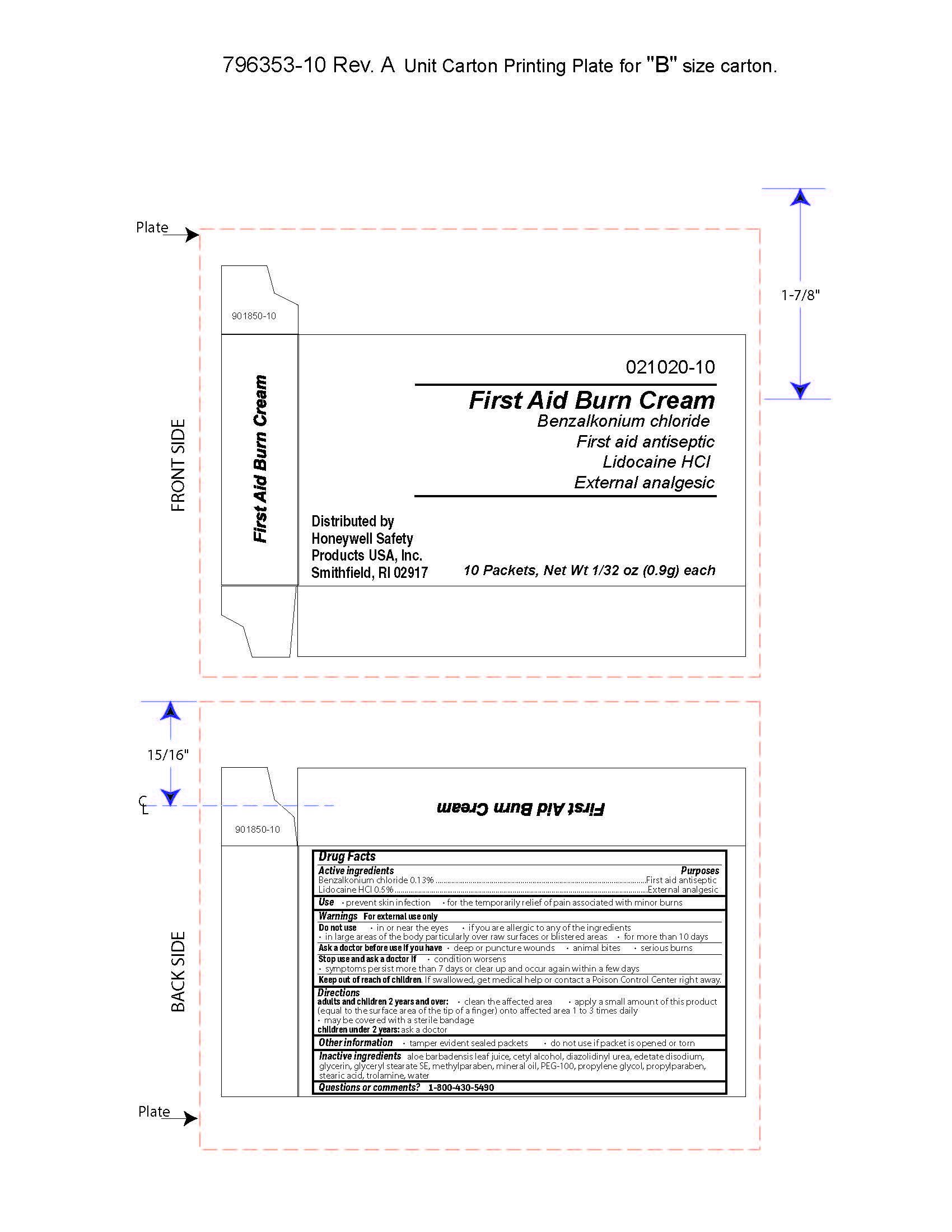
Burn Cream
Active ingredient
Benzalkonium chloride 0.13%
Lidocaine hydrochloride 0.5%
Burn Cream
Purpose
First aid antiseptic
External analgesic
Burn Cream
Uses
- prevent skin infection
- for temporary relief of pain associated with miinor burns
Burn Cream
Warnings
For external use only
Do not use
- in or near the eyes
- if you are allergic to any of the ingredients
- in large areas of the body particularly over raw surfaces or blistered areas
- for more than 10 days
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition persists
- symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of chidren
If swallowed, get medical help or contact a Poison Control Center right away.
Burn Cream
Directions
- adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product (equal to the area of the tip of finger) onto the affected area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: ask a doctor
Burn Cream
Other information
- tamper evident sealed packets
- do not use if packet is opened or torn
Burn Cream
Inactive ingredients
aloe barbadensis juice, cetyl alcohol, diazolidinyl urea, edetate disodium, glycerin, glyceryl stearate SE, methylparaben, mineral oil, PEG-100, propylene glycol, propylparaben, stearic acid, trolamine, water
Burn Cream
Questions
1-800-430-5490
Neomycin
Active ingredient
Neonycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Neomycin
Purpose
First aid antibiotic
Neomycin
Uses
first aid to help prevent infection in:
Neomycin
Warnings
For external use only
Neomycin
Do not use
- in the eyes
- over large areas of the body
Neomycin
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Neomycin
Stop use and ask a doctor if:
- the condition persists or gets worse
- a rash or other allergic reaction develops
- you need to use longer than 1 week
Neomycin
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Neomycin
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Neomycin
Other information
Store at 15
o to 25
o C (59
o to 77
oF)
Neomycin
Inactive ingredients
petrolatum
Neomycin
Questions
1-800-430-5490
4044
Z019733-0020L Kit Contents
FIRST AID GUIDE ASHI
GAUZE CLEAN-WRAP BDGE N/S 2"
PVP PREP PADS MEDIUM 100/BX
SCISSOR BDGE 4" RED PLS HDL
FIRST AID CREAM 1.0GR PKT EACH
TAPE ADHESIVE 1/2 X 2.5 125133
POUCH NEOMYCIN ANTIBIOTIC .9 G
ADH BNDG PLASTIC EX-LG 4"X 2"
GAUZE PADS 3"X3" 12PLY
PLASTIC BANDAGE 1" X 3"
4045
019733-0020L Kit Contents
FIRST AID GUIDE ASHI
GAUZE CLEAN-WRAP BDGE N/S 2"
PVP PREP PADS MEDIUM 100/BX
SCISSOR BDGE 4" RED PLS HDL
FIRST AID CREAM 1.0GR PKT EACH
TAPE ADHESIVE 1/2 X 2.5 125133
POUCH NEOMYCIN ANTIBIOTIC .9 G
ADH BNDG PLASTIC EX-LG 4"X 2"
GAUZE PADS 3"X3" 12PLY
PLASTIC BANDAGE 1" X 3"
Honeywell PVP Wipes
Burn Cream
Principal Display Panel
Neomycin
Principal Display Panel

4044 Kit Label
Z019733-0019L

4045 Kit Label
019733-0020L





