4047 FIST AID KIT- 4047 first aid kit
4048 FIST AID KIT- 4048 first aid kit
Honeywell Safety Products USA, Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Alcohol Wipes
Active ingredient
Isopropyl alcohol 70%
Alcohol Wipes
Purpose
First aid antiseptic
Alcohol Wipes
Uses
first aid to help prevent infection in minor cuts, scrapes, and burns
Alcohol Wipes
Warnings
For external use only
Flammable, keep away from fire and flame
Do not use
- in or near eyes
- over large areas of the body
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
When using this product
- do not use longer than 1 week unless directed by a doctor
Stop use and consult a doctor if
- condition persists or gets worse
Keep out of the reach of children
If swallowed, get medical help or contact a Poison Control center right away
Alcohol Wipes
Directions
- clean the affected area
- may be covered with a sterile bandage
- apply wipe to affeted are 1 to 3 times daily
- discard wipe after single use
Alcohol Wipes
Other information
- store at room temperature 15
o to 25
o C (59
o to 77
oF)
- do not use if packet is torn or opened
Alcohol Wipes
Inactive ingredient
water
Alcohol Wipes
Questions
1-800-430-5490
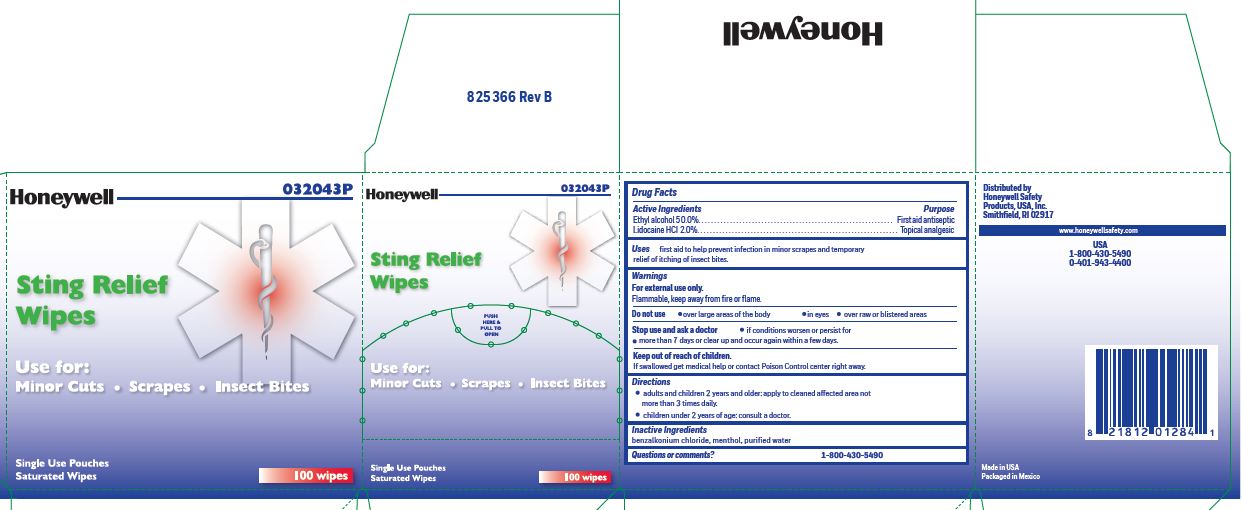
Sting Relief
Active ingredient
Ethyl alcohol 50.0%
Lidocaine hydrochloride 2.0%
Sting Relief
Purpose
First aid antiseptic
Topical analgesic
Sting Relief
Uses
- prevent infection in minor scrapes, and temporary rellief of itching of insect bites
Sting Relief
Warnings
For external use only
Flammable, keep away from fire or flame
Sting Relief
Do not use
- in large quantities
- over large areas of the body
- in eyes
- over raw or blistered areas
Stop use and ask a doctor
- if condition worsens or persists for more than 7 days or clear up and occur again within a few days
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Sting Relief
Directions
- adults and children 2 years and older: apply to cleaned affected area not more than 3 times daily
- children under 2 years of age: consult a doctor
Sting Relief
Other information
store at room temperature 15
o to 25
oC (59
o to 77
o F)
Sting Relief
Inactive ingredient
benzalkonium chloride, menthol, purified water
Sting Relief
Questions
1-800-430-5490
4047
Z019741-0028L Kit Contents
WIPE ALCOHOL PREP IPA 70% (DUKAL)
DISPOSABLE RAZOR
SAFETEC STING RELIEF WIPES BULK
PLASTIC BANDAGE 1" X 3"
SNAKE BITE PUMP
4048
019741-0028L Kit Contents
WIPE ALCOHOL PREP IPA 70% (DUKAL)
DISPOSABLE RAZOR
SAFETEC STING RELIEF WIPES BULK
PLASTIC BANDAGE 1" X 3"
SNAKE BITE PUMP
Alcohol wipes 10 ct

Sting Relief
Principal Display Panel
4047 Kit Label

4048 Kit Label




