FLEET- mineral oil and witch hazel
C.B. Fleet Company, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
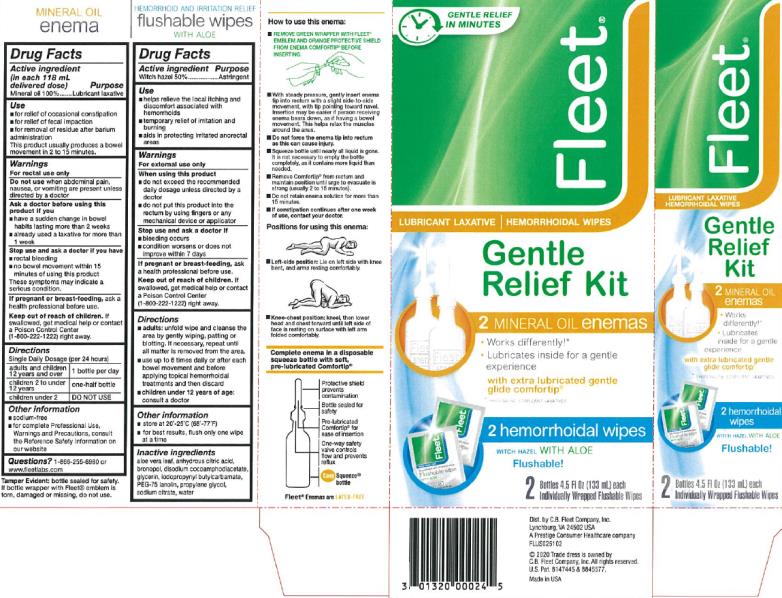
Fleet Gentle Relief Kit
Uses
- for relief of occasional constipation
- for relief of fecal impaction
- for removal of residue after barium administration
This product usually produces a bowel movement in 2 to 15 minutes.
Warnings
For rectal use only
Ask a doctor before using this product if you
- have a sudden change in bowel habits lasting more than 2 weeks
- already used a laxative for more than 1 week
Directions
Single Daily Dosage (per 24 hours)
| adults and children 12 years and over | 1 bottle per day |
| children 2 to under 12 years | one-half bottle |
| children under 2 | DO NOT USE |
Other information
- sodium-free
- for complete Professional Use, Warnings and Precautions, consult the Reference Safety Information on our website
Questions?
1-866-255-6960 or www.fleetlabs.com
Tamper Evident bottle sealed for safety. If bottle wrapper with Fleet® emblem is torn, damaged or missing, do not use.
Hemorrhoidal Relief Wipes
Drug Facts
Uses
- helps relieve the local itching and discomfort associated with hemorrhoids
- temporary relief of irritation and burning
- aids in protecting irritated anorectal areas
Warnings
For external use only
Directions
- adults: unfold wipe and cleanse the area by gently wiping, patting or blotting. if necessary, repeat until all matter is removed from the area.
- use up to 6 times daily or after each bowel movement and before applying topical hemorrhoidal treatments and then discard
- children under 12 years of age: consult a doctor
Other information
- store at 20º-25ºC (68º-77ºF)
- for best results, flush only one or two wipes at a time
| FLEET
mineral oil and witch hazel kit |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - C.B. Fleet Company, Inc. (003119054) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nice-Pak Products, Inc. | 067900167 | manufacture(0132-0705) | |
Revised: 11/2022
Document Id: ecaa652a-d6c5-321f-e053-2995a90aff6a
Set id: 7b5dbbed-8ada-49a1-8773-1559048bfa1c
Version: 4
Effective Time: 20221104
C.B. Fleet Company, Inc.