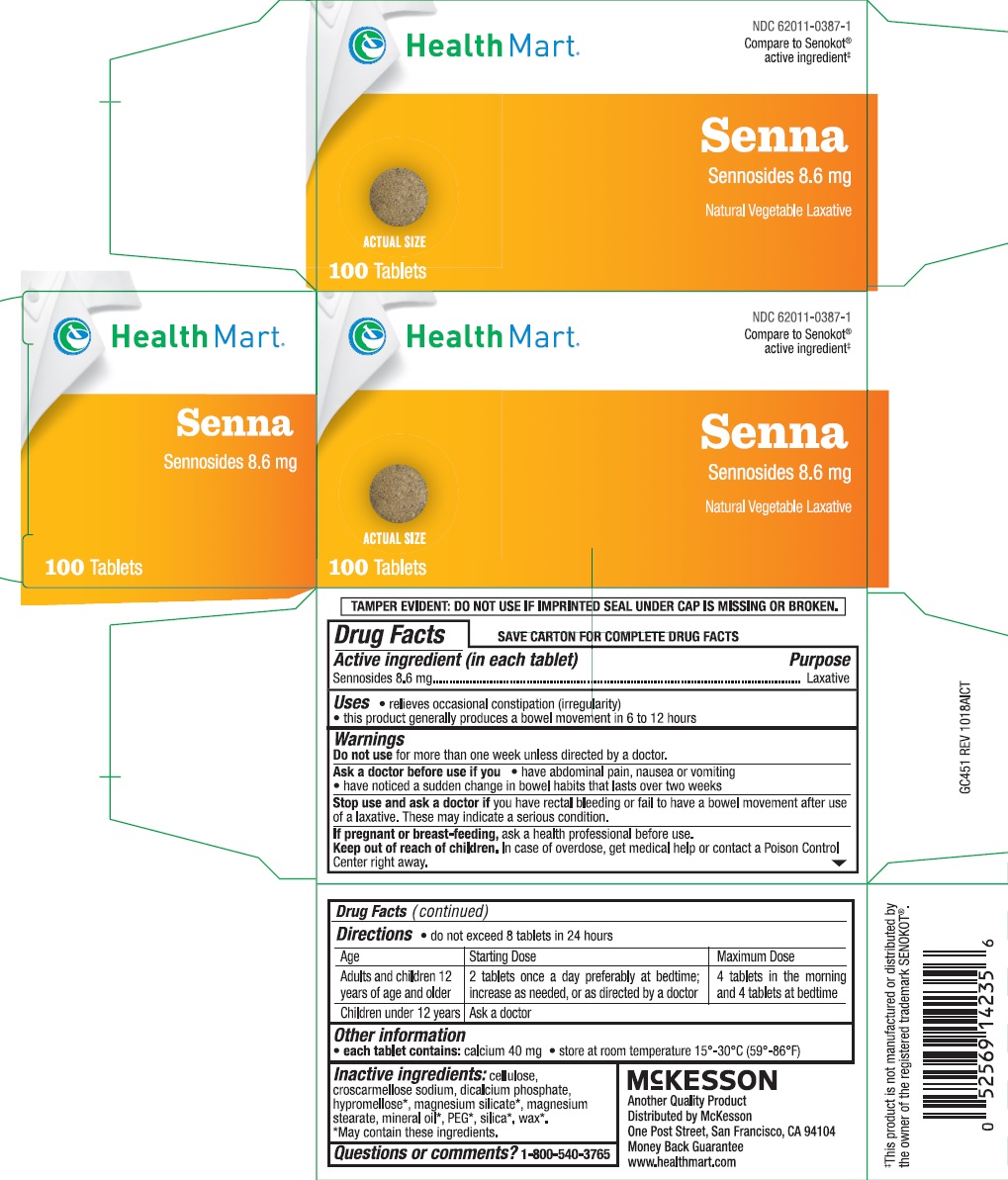
SENNA- sennosides tablet
STRATEGIC SOURCING SERVICES LLC
----------
healthmart 451
Uses
- this product generally produces a bowel movement in 6 to 12 hours
- relieves occasional constipation (irregularity)
Warnings
Do not use for more than one week unless directed by a doctor.
Ask a doctor before use if you
- have abdominal pain, nausea or vomiting
- have noticed a sudden change in bowel habits that lasts over two weeks
Stop use and ask a doctor if you have rectal bleeding or fail to have a bowel movement after use if a laxative. These may indicate a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Directions
- do not exceed 8 tablets in 24 hours
| Age | Starting Dose | Maximum Dose |
|---|---|---|
| adults and children 12 years of age and older | 2 tablets once a day preferably at bedtime; increase as needed, or as directed by a doctor | 4 tablets in the morning and 4 tablets at bedtime |
| children under 12 years | ask a doctor |
| SENNA
sennosides tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - STRATEGIC SOURCING SERVICES LLC (116956644) |
| Registrant - Geri-Care Pharmaceutical Corp (611196254) |
Revised: 10/2023
Document Id: 07da5ac2-03f3-e68e-e063-6294a90a46ed
Set id: 7ab79125-4394-2bc3-e053-2a91aa0a6307
Version: 4
Effective Time: 20231016
STRATEGIC SOURCING SERVICES LLC