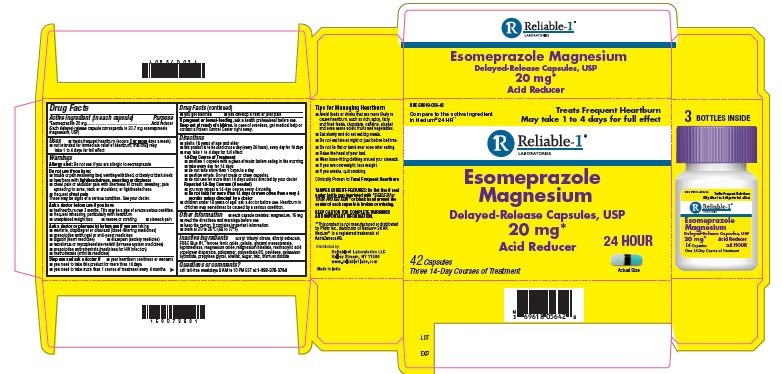
Label: ESOMEPRAZOLE capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 69618-056-14, 69618-056-42 - Packager: Reliable 1 Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 9, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each capsule)
- Purpose
- Uses
-
Warnings
Allergy alert: Do not use if you are allergic to esomeprazole
Do not use if you have:
trouble or pain swallowing food, vomiting with blood, or bloody or black stools
heartburn with lightheadedness, sweating or dizziness
chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
frequent chest pain
These may be sings of a serious condition. See your doctor.
- ASK DOCTOR
-
ASK DOCTOR/PHARMACIST
Ask a doctor or pharmacist before use if you are taking
warfarin, clopifogrel or cilostazol (blood-thinning medicines)
prescription antifungal or anti-yeast medications
digozin (heart medicine)
diazepam (anxiety medicine)
tacrolimus or mycophenolate mofetil (immune system medicines)
prescription antiretovirals (medicines for HIV infections)
methotrexate (arthritis medicine)
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
adults 18 years of age and older
this prodcut is to be used once a day (every 24 hours), every day for 14 days
make take 1 to 4 days for full effect
14-Day Course of Treatment
swallow 1 capsule with a glass of water before eating in the morning
take every day for 14 days
do not take more than 1 capsule a day
swallow whole. Do not crush or chew capsules.
do not use of more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
you may repeart a 14-day course every 4 months
do not take for more than 14 days or more often than every 4 mounths unless directed by a doctor
children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be cuased by a serious condition.
- Other Information
-
Inactive ingredients
acetyl tributyl citrate, dibutyl sebacate, FD&C Blue #1, ferroso ferric oxide, gelatin, glyceryl monostearate, hypromellose, magnesium odixe, magnesium stearate, methacrylic acid copolymer dispersion, poloxamer, polysorbate 80, povidone, potassium hydroxide, propylene glycol, shellac, sugar, talc, titanium dioxide
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ESOMEPRAZOLE
esomeprazole capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69618-056 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESOMEPRAZOLE MAGNESIUM (UNII: R6DXU4WAY9) (ESOMEPRAZOLE - UNII:N3PA6559FT) ESOMEPRAZOLE 20 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) SHELLAC (UNII: 46N107B71O) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) MAGNESIUM OXIDE (UNII: 3A3U0GI71G) DIBUTYL SEBACATE (UNII: 4W5IH7FLNY) FERROSOFERRIC OXIDE (UNII: XM0M87F357) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLOXAMER 188 (UNII: LQA7B6G8JG) ACETYLTRIBUTYL CITRATE (UNII: 0ZBX0N59RZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color yellow (Gelatin capsule shell with light blue cap and dark blue body) Score no score Shape OVAL (Capsule) Size 9mm Flavor Imprint Code RDY;327 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69618-056-42 3 in 1 CARTON 11/01/2018 1 NDC:69618-056-14 14 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207673 11/01/2018 Labeler - Reliable 1 Laboratories (079718111)