Label: SKIN LIGHTENING RODAN FIELDS- hydroquinone liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 14222-1615-1, 14222-1615-2 - Packager: Rodan & Fields, LLC.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 24, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Hydroquinone 2%
Keep out of reach of children
if swallowed, get medical help or contact a poison control center immediately.
avoid contact with eyes.
some user of this product may experience a mild skin irritation. if skin irritation becomes severe , stop use and consult a physician.
do not use if pregnant or nursing.
do not use on children under 12 years of age unless directed by a physician.apply evenly to the entire face with a fresh gauze pad, avoiding the eye area. do not rinse off, allow to dry. use once daily and increase to twice daily as tolerated.
Adult: apply a small amount as a think layer in affected area once or twice daily, or use as directed by a physician . if no improvement is seen after three months of treatment, use of this product should be discontinued . lightening effect of this product may not be noticeable when used on very dark skin.
children under 12 years of age: do not use unless directed by a physician.
Water (aqua), Ethoxydiglycol, Hamamelis Virgiana ( Witch Hazel) Water, Methylpropanediol, Alcohol Denat, Polysorbate 20, Butylene Glycol, Aminopropyl Ascorbyl Phosphate, Foeniculum Vulgare ( Fennel) Seed Extract, Magnesium Ascorbyl Phosphate, Arctostaphylos Uva Ursi (leaf) Extract, PEG-12, Dimethicone, Arnica Montana Flower Extract, Zinc Phenolsulfonate, Kojic Acid, Citrus Medica Limonum ( Lemon) Peel Extract, Sodium Salicylate, Citric Acid, Sodium Sulfite, Sodium Metabisulfite, DMDM Hydantoin, Fragrance, Yellow 5, Yellow 6
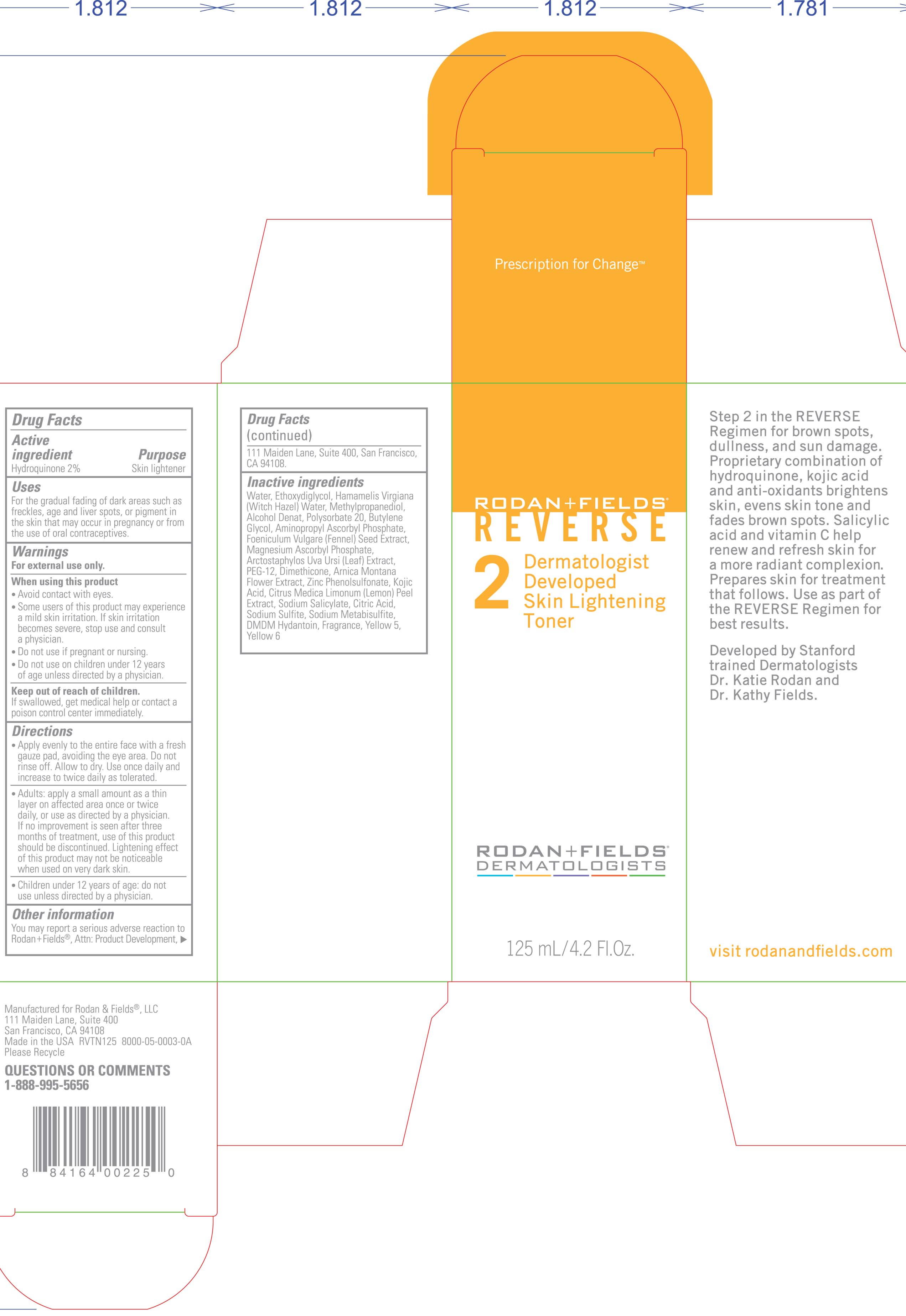
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SKIN LIGHTENING RODAN FIELDS
hydroquinone liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-1615 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROQUINONE (UNII: XV74C1N1AE) (HYDROQUINONE - UNII:XV74C1N1AE) HYDROQUINONE 2 mL in 100 mL Inactive Ingredients Ingredient Name Strength DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) WITCH HAZEL (UNII: 101I4J0U34) METHYLPROPANEDIOL (UNII: N8F53B3R4R) POLYSORBATE 20 (UNII: 7T1F30V5YH) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) FENNEL SEED (UNII: G3QC02NIE6) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) ARCTOSTAPHYLOS UVA-URSI LEAF (UNII: 3M5V3D1X36) POLYETHYLENE GLYCOL 600 (UNII: NL4J9F21N9) DIMETHICONE (UNII: 92RU3N3Y1O) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) ZINC PHENOLSULFONATE (UNII: 4O71YT5YB5) KOJIC ACID (UNII: 6K23F1TT52) LEMON PEEL (UNII: 72O054U628) SODIUM SALICYLATE (UNII: WIQ1H85SYP) SODIUM SULFITE (UNII: VTK01UQK3G) SODIUM METABISULFITE (UNII: 4VON5FNS3C) DMDM HYDANTOIN (UNII: BYR0546TOW) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-1615-2 1 in 1 BOX 1 NDC:14222-1615-1 125 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part358A 06/06/2011 Labeler - Rodan & Fields, LLC. (051659584) Registrant - Cosmetic Enterprises Ltd (017701475) Establishment Name Address ID/FEI Business Operations Cosmetic Enterprises Ltd 017701475 manufacture


