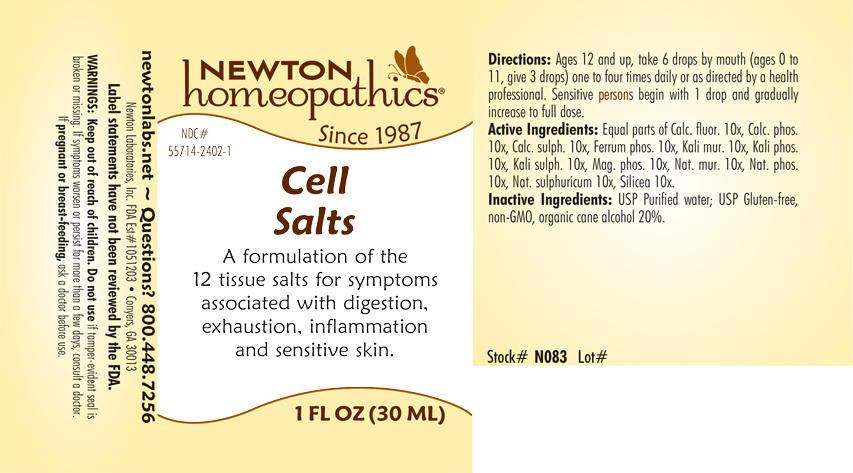
Label: CELL SALTS- calc. fluor., calc. phos., calc.sulph., ferrum phosphoricum, kali mur., kali phos., kali sulph., mag. phos., nat. mur., nat. phos., nat.sulphuricum, silicea liquid
- NDC Code(s): 55714-2402-0, 55714-2402-1, 55714-2402-2
- Packager: Newton Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 13, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- OTC - KEEP OUT OF REACH OF CHILDREN SECTION
- OTC - PREGNANCY OR BREAST FEEDING SECTION
- WARNINGS SECTION
- QUESTIONS SECTION
- INACTIVE INGREDIENT SECTION
- OTC - PURPOSE SECTION
- OTC - ACTIVE INGREDIENT SECTION
- DOSAGE & ADMINISTRATION SECTION
- INDICATIONS & USAGE SECTION
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
CELL SALTS
calc. fluor., calc. phos., calc.sulph., ferrum phosphoricum, kali mur., kali phos., kali sulph., mag. phos., nat. mur., nat. phos., nat.sulphuricum, silicea liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55714-2402 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 10 [hp_X] in 1 mL TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (PHOSPHATE ION - UNII:NK08V8K8HR) TRIBASIC CALCIUM PHOSPHATE 10 [hp_X] in 1 mL CALCIUM SULFATE ANHYDROUS (UNII: E934B3V59H) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SULFATE ANHYDROUS 10 [hp_X] in 1 mL FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 10 [hp_X] in 1 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 10 [hp_X] in 1 mL POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) (PHOSPHATE ION - UNII:NK08V8K8HR) POTASSIUM PHOSPHATE, DIBASIC 10 [hp_X] in 1 mL POTASSIUM SULFATE (UNII: 1K573LC5TV) (SULFATE ION - UNII:7IS9N8KPMG) POTASSIUM SULFATE 10 [hp_X] in 1 mL MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 10 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 10 [hp_X] in 1 mL SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE 10 [hp_X] in 1 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 10 [hp_X] in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 10 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55714-2402-0 15 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 11/07/2018 01/31/2020 2 NDC:55714-2402-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 11/07/2018 3 NDC:55714-2402-2 50 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 11/07/2018 01/31/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/07/2018 Labeler - Newton Laboratories, Inc. (788793610) Registrant - Newton Laboratories, Inc. (788793610) Establishment Name Address ID/FEI Business Operations Newton Laboratories, Inc. 788793610 manufacture(55714-2402)