Label: tepanil- diethylpropion hydrochloride tablet, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 0536-3701-01, 0536-3701-02 - Packager: Qualitest Products, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated July 26, 2006
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
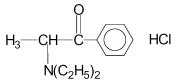
Diethylpropion hydrochloride, a sympathomimetic agent. Chemically, diethylpropion hydrochloride is 1-phenyl-2-diethylamino-1-propanone hydrochloride.
Structural formula: C13H18NO HCl
Molecular weight: 241.76
Each tablet contains diethylpropion hydrochloride 75 mg in a controlled-release formulation. Diethylpropion hydrochloride tablets also contain: carbomer, mannitol, povidone, tartaric acid and zinc stearate.
Diethylpropion hydrochloride is dispersed in a hydrophilic matrix. On exposure to water the diethylpropion hydrochloride is released at a relatively uniform rate as a result of slow hydration of the matrix. The result is controlled release of the anorexic agent.
-
CLINICAL PHARMACOLOGY
Diethylpropion hydrochloride is a sympathomimetic amine with some pharmacologic activity similar to that of the prototype drugs of this class used in obesity, the amphetamines. Actions include some central nervous system stimulation and elevation of blood pressure. Tolerance has been demonstrated with all drugs of this class in which these phenomena have been looked for.
Drugs of this class used in obesity are commonly known as “anorectics” or “anorexigenics”. It has not been established, however, that the action of such drugs in treating obesity is primarily one of appetite suppression. Other central nervous system actions or metabolic effects may be involved, for example.
Adult obese subjects instructed in dietary management and treated with “anorectic” drugs lose more weight on the average than those treated with placebo and diet, as determined in relatively short-term clinical trials.
The magnitude of increased weight loss of drug-treated patients over placebo-treated patients averages some fraction of a pound a week. However, individual weight loss may vary substantially from patient to patient. The rate of weight loss is greatest in the first weeks of therapy for both drug and placebo subjects and tends to decrease in succeeding weeks. The possible origins of the increased weight loss due to the various drug effects are not established. The amount of weight loss associated with the use of an “anorectic” drug varies from trial to trial, and the increased weight loss appears to be related in part to variables other than the drug prescribed, such as the physician/investigator relationship, the population treated, and the diet prescribed. Studies do not permit conclusions as to the relative importance of the drug and non-drug factors on weight loss.
The natural history of obesity is measured in years, whereas most studies cited are restricted to a few weeks duration. Thus, the total impact of drug-induced weight loss over that of diet alone is unknown.
Diethylpropion is rapidly absorbed from the GI tract after oral administration and is extensively metabolized through a complex pathway of biotransformation involving N-dealkylation and reduction. Many of these metabolites are biologically active and may participate in the therapeutic action of these drugs. Due to the varying lipid solubilities of these metabolites, their circulating levels are affected by urinary pH. Diethylpropion and/or its active metabolites are believed to cross the blood-brain barrier and the placenta (see Labor and Delivery).
Diethylpropion and its metabolites are excreted mainly by the kidney. It has been reported that between 75-106% of the dose is recovered in the urine within 48 hours after dosing. Using a phosphorescence assay that is specific for basic compounds containing a benzoyl group, the plasma half-life of the aminoketone metabolites is estimated to be between 4 to 6 hours.
The controlled-release characteristics of Diethlpropion have been demonstrated by studies in humans in which plasma levels of diethylpropion-related material were measured by phosphorescence analysis. Plasma levels obtained with the 75 mg Diethylpropion formulation administered once daily indicated a more gradual release than the standard formulation, however, it has not been shown superior in effectiveness to the same dosage of the standard, non-controlled-release formulation.
-
INDICATIONS AND USAGE
Diethylpropion hydrochloride tablets are indicated in the management of exogenous obesity as a short-term adjunct (a few weeks) in a regiment of weight reduction based on caloric restriction. The usefulness of agents of this class should be measured against possible risk factors inherent in their use such as those described (see CLINICAL PHARMACOLOGY).
-
CONTRAINDICATIONS
Diethylpropion hydrochloride should not be used in patients with advanced arteriosclerosis, hyperthyroidism, known hypersensitivity or idiosyncrasy to the sympathomimetic amines, glaucoma, severe hypertension, a history of drug abuse, or those in an agitated state (see PRECAUTIONS).
Diethylpropion hydrochloride should not be given during, or within fourteen days following, the administration of monoamine oxidase inhibitors; hypertensive crises may result.
- WARNINGS
-
PRECAUTIONS
General Precautions:
Caution is to be exercised in prescribing Diethylpropion hydrochloride for patients with hypertension or with symptomatic cardiovascular disease, including arrhythmias. They should not be administered to patients with severe hypertension. Reports suggest that Diethylpropion hydrochloride may increase convulsions in some epileptics. Therefore, epileptics receiving them should be carefully monitored. Titration of dose or discontinuance of drug may be necessary. The least amount feasible should be prescribed or dispensed at one time to minimize the possibility of an overdose.
Information for Patients:
Diethylpropion hydrochloride may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; ambulatory patients should therefore by cautioned accordingly. When central nervous system-active agents are used, consideration must always be given to the possibility of adverse interactions with alcohol.
Drug Interactions:
Antidiabetic drug requirements, i.e., insulin, may be altered in association with the use of Diethylpropion hydrochloride and the concomitant dietary regimen. Concurrent use with general anesthetics may result in arrhythmias. The presser effects of diethylpropion and those of other drugs may be additive when the drugs are used concomitantly; conversely, diethylpropion may interfere with antihypertensive drugs, i.e., guanethidine, a-methyldopa. Concurrent use of phenothiazines may antagonize the anorectic effect of diethylpropion.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Diethylpropion hydrochloride has not been evaluated for carcinogenicity, mutagenicity, or impairment of fertility.
Pregnancy: Teratogenic Effects: - Pregnancy Category B:
Reproduction studies have been performed in rats at doses up to nine times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to diethylpropion hydrochloride. However, no adequate and well-controlled studies have been conducted in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy: Nonteratogenic Effects:
Abuse during pregnancy may result in withdrawal symptoms in the neonate.
Labor and Delivery:
Diethylpropion hydrochloride has no recognized use during labor and delivery, and its effect during these processes are unknown. Diethylpropion and /or its active metabolites are believed to cross the placenta.
-
ADVERSE REACTIONS
The following adverse effects, listed by organ system, have been associated with the use of Diethylpropion hydrochloride.
Cardiovascular:
Precordial pain, arrhythmia, ECG changes (one published report described T-wave changes in the ECG of a healthy young male after ingestion of diethylpropion hydrochloride), tachycardia, elevation of blood pressure, palpitation.
Central Nervous System:
In a few epileptics an increase in convulsive episodes has been reported: dyskinesia, blurred vision, overstimulation, nervousness, restlessness, dizziness, jitteriness, insomnia, anxiety, euphoria, depression, dysphoria, tremor, mydriasis, drowsiness, malaise, headache, and rarely, psychotic episodes at recommended doses.
-
DRUG ABUSE AND DEPENDENCE:
Abuse:
Diethylpropion has some chemical and pharmacologic similarities to the amphetamines and other related stimulant drugs that have been extensively abused. The possibility of abuse should be kept in mind when evaluating the desirability of including a drug as part of a weight reduction program. Abuse of amphetamines and related drugs may be associated with varying degrees of psychologic dependence and social dysfunction which, in the case of certain drugs, may be severe. There are reports of patients who have increased the dosage to many times that recommended.
Dependence:
There have been reports of subjects becoming psychologically dependent on diethylpropion. Abrupt cessation following prolonged high dosage administration results in extreme fatigue and mental depression; changes are also noted on the sleep EEG. Manifestations of chronic intoxication with anorectic drugs include severe dermatoses, marked insomnia, irritability, hyperactivity, and personality changes. The most severe manifestation of chronic intoxication is psychosis, often clinically indistinguishable from schizophrenia.
-
OVERDOSAGE
The reported oral LD50, for diethylpropion hydrochloride in mice is 620 mg/kg. In rats 250 mg/kg and in dogs 225 mg/kg.
Symptoms:
Manifestations of acute overdosage include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states.
Fatigue and depression usually follow the central stimulation.
Cardiovascular effects include arrhythmias, hypertension or hypotension, and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea, and abdominal cramps. Overdose of pharmacologically similar compounds has resulted in fatal poisoning, usually terminating in convulsions and coma.
Treatment:
Management of acute Diethylpropion hydrochloride intoxication is largely symptomatic and includes lavage and sedation with a barbiturate. Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendation in this regard: Intravenous phentolamine (Regitine®) has been suggested on pharmacologic grounds for possible acute, severe hypertension. If this complicates diethylpropion hydrochloride overdosage.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Diethylpropion hydrochloride 75 mg tablets are oval, white, domed, imprinted “2690V” on one side and plain on the other.
Diethylpropion hydrochloride 75 mg tablets are supplied in bottles of 100 and 250.
Dispense in a tight, light-resistant container as defined in the USP with a child-resistant closure. Store at controlled room temperature, 15°- 30°C (59°- 86°F).
- CAUTION:
-
INGREDIENTS AND APPEARANCE
TEPANIL
diethylpropion hydrochloride tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0536-3701 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength diethylpropion hydrochloride (UNII: 19V2PL39NG) (diethylpropion - UNII:Q94YYU22B8) 75 mg Inactive Ingredients Ingredient Name Strength carbomer () mannitol (UNII: 3OWL53L36A) povidone () tartaric acid (UNII: W4888I119H) zinc stearate (UNII: H92E6QA4FV) Product Characteristics Color WHITE (WHITE) Score no score Shape OVAL (OVAL) Size 12mm Flavor Imprint Code 2690V Contains Coating false Symbol false Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0536-3701-01 100 in 1 BOTTLE 2 NDC:0536-3701-02 250 in 1 BOTTLE Labeler - Qualitest Products, Inc.