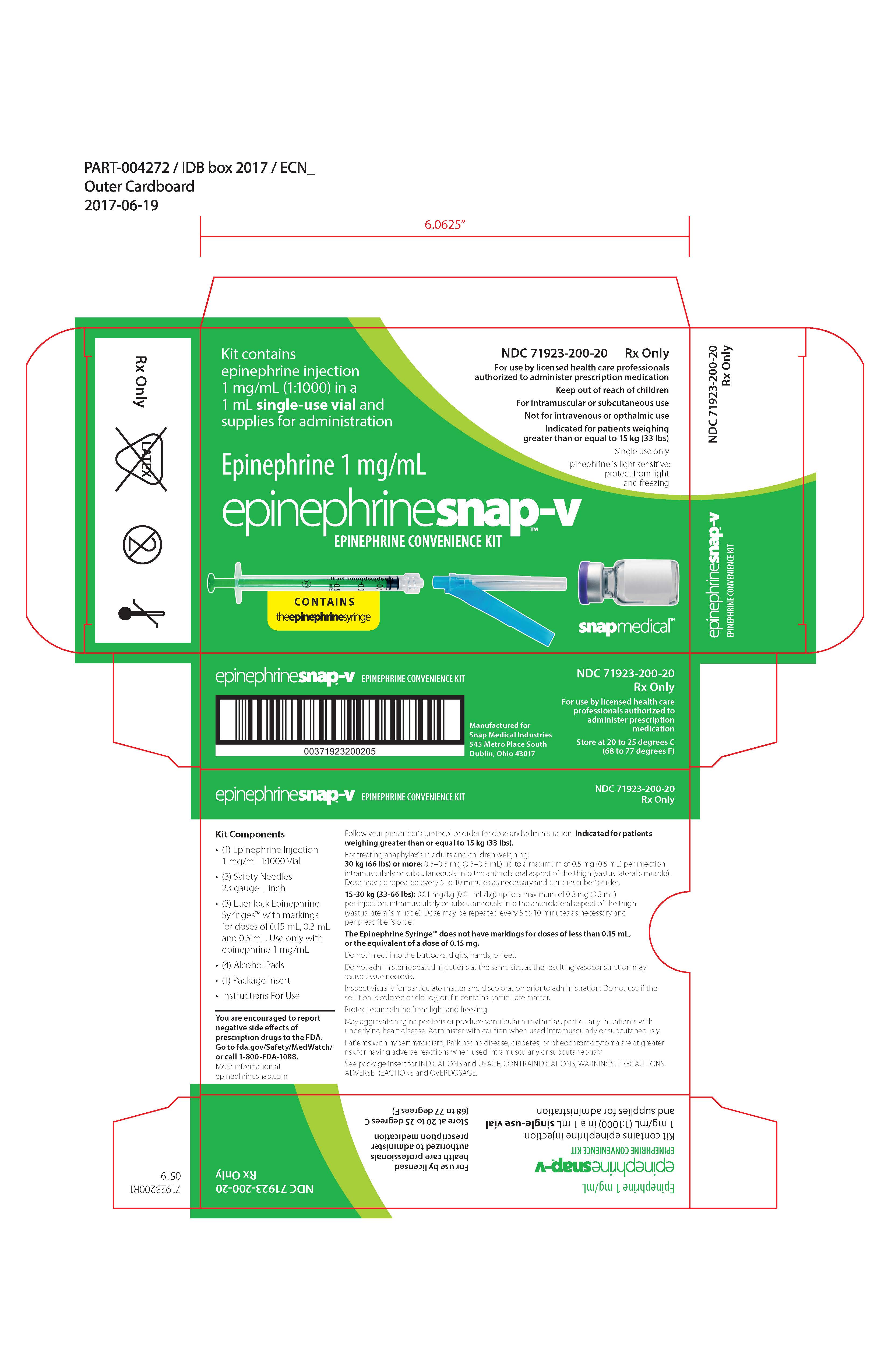
Label: EPINEPHRINESNAP-V- epinephrine convenience kit kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 42023-159-25, 68599-5804-1, 71923-200-20 - Packager: Snap Medical Industries
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ADRENALIN safely and effectively. See full prescribing information for ADRENALIN.
ADRENALIN (epinephrine injection) 1 mg/mL,
for intramuscular and subcutaneous use
Initial U.S. Approval: 1939RECENT MAJOR CHANGES
Dosage and Administration (2) 06/19
INDICATIONS AND USAGE
Adrenalin ® is a non-selective alpha and beta adrenergic agonist indicated for:
- Emergency treatment of allergic reactions (Type 1), including anaphylaxis ( 1)
DOSAGE AND ADMINISTRATION
-
Anaphylaxis:
- Adults and Children 30 kg (66 lbs) or more: 0.3 to 0.5 mg (0.3 to 0.5 mL) intramuscularly or subcutaneously into anterolateral aspect of the thigh every 5 to 10 minutes as necessary ( 2)
- Children 30 kg (66 lbs) or less: 0.01 mg/kg (0.01 mL/kg), up to 0.3 mg (0.3 mL), intramuscularly or subcutaneously into anterolateral aspect of the thigh every 5 to 10 minutes as necessary ( 2)
DOSAGE FORMS AND STRENGTHS
Injection: 1 mg/mL, 1 mL single-use vials and 30 mL multiple-dose vials ( 3)
CONTRAINDICATIONS
None ( 4)
WARNINGS AND PRECAUTIONS
- Do not inject into buttocks, digits, hands, or feet ( 5.1)
- Rare cases of serious skin and soft tissue infections have been reported following epinephrine injection. Advise patients to seek medical care if they develop signs or symptoms of infection. ( 5.2)
- May aggravate angina pectoris or produce ventricular arrhythmias, particularly in patients with underlying heart disease, administer with caution when used intramuscularly or subcutaneously ( 5.3)
- Patients with hyperthyroidism, Parkinson's disease, diabetes, and pheochromocytoma are at greater risk of having adverse reactions when used intramuscularly or subcutaneously ( 5.3)
- Presence of sulfite in this product should not deter use for anaphylaxis ( 5.4)
ADVERSE REACTIONS
Common adverse reactions to systemically administered epinephrine include anxiety, apprehensiveness, restlessness, tremor, weakness, dizziness, sweating, palpitations, pallor, nausea and vomiting, headache, and respiratory difficulties. Arrhythmias, including fatal ventricular fibrillation, rapid rises in blood pressure producing cerebral hemorrhage, and angina have occurred ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Par Pharmaceutical at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Sympathomimetic agents: possible additive effects ( 7)
- Cardiac glycosides, halogenated hydrocarbon anesthetics, or diuretics: observe for development of cardiac arrhythmias ( 7)
- Tricyclic antidepressants, MAO inhibitors, levothyroxine sodium, and certain antihistamines: potentiate effects of epinephrine ( 7)
- Beta-adrenergic blocking drugs: antagonize the cardiostimulating and bronchodilating effects of epinephrine ( 7)
- Alpha-adrenergic blocking drugs: antagonize the vasoconstricting and hypertensive effects of epinephrine ( 7)
- Ergot alkaloids may reverse the pressor response to epinephrine ( 7)
USE IN SPECIFIC POPULATIONS
Elderly patients and pregnant women may be at greater risk of developing adverse reactions when epinephrine is administered parenterally ( 8.1, 8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
RECENT MAJOR CHANGES
1 ADRENALIN ® INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Incorrect Locations of Injection
5.2 Serious Infections at the Injection Site
5.3 Disease Interactions
5.4 Allergic Reactions Associated with Sulfite
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 ADRENALIN
® INDICATIONS AND USAGE
Adrenalin ® is available as a single-use 1 mL vial and a multiple-use 30 mL vial for intramuscular and subcutaneous use.
Emergency treatment of allergic reactions (Type I), including anaphylaxis, which may result from allergic reactions to insect stings, biting insects, foods, drugs, sera, diagnostic testing substances and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis. The signs and symptoms associated with anaphylaxis include flushing, apprehension, syncope, tachycardia, thready or unobtainable pulse associated with hypotension, convulsions, vomiting, diarrhea and abdominal cramps, involuntary voiding, airway swelling, laryngospasm, bronchospasm, pruritus, urticaria or angioedema, swelling of the eyelids, lips, and tongue.
-
2 DOSAGE AND ADMINISTRATION
Inject Adrenalin ® intramuscularly or subcutaneously into the anterolateral aspect of the thigh, through clothing if necessary. When administering to a child, to minimize the risk of injection related injury, hold the leg firmly in place and limit movement prior to and during an injection. The injection may be repeated every 5 to 10 minutes as necessary. For intramuscular administration, use a needle long enough (at least 1/2 inch to 5/8 inch) to ensure the injection is administered into the muscle. Monitor the patient clinically for the severity of the allergic reaction and potential cardiac effects of the drug, with repeat doses titrated to effect. Do not administer repeated injections at the same site, as the resulting vasoconstriction may cause tissue necrosis.
Inspect visually for particulate matter and discoloration prior to administration. Do not use if the solution is colored or cloudy, or if it contains particulate matter.
Adults and Children 30 kg (66 lbs) or more: 0.3 to 0.5 mg (0.3 mL to 0.5 mL) of undiluted Adrenalin ® administered intramuscularly or subcutaneously in the anterolateral aspect of the thigh, up to a maximum of 0.5 mg (0.5 mL) per injection, repeated every 5 to 10 minutes as necessary. Monitor clinically for reaction severity and cardiac effects.
Children less than 30 kg (66 lbs): 0.01 mg/kg (0.01 mL/kg) of undiluted Adrenalin ® administered intramuscularly or subcutaneously in the anterolateral aspect of the thigh, up to a maximum of 0.3 mg (0.3 mL) per injection, repeated every 5 to 10 minutes as necessary. Monitor clinically for reaction severity and cardiac effects.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Incorrect Locations of Injection
Injection into the anterolateral aspect of the thigh (vastus lateralis muscle) is the most appropriate location for administration because of its location, size, and available blood flow. Injection into (or near) smaller muscles, such as in the deltoid, is not recommended due to possible differences in absorption associated with this use.
Do not administer repeated injections of epinephrine at the same site, as the resulting vasoconstriction may cause tissue necrosis.
Do not inject into buttock. Injection into the buttock may not provide effective treatment of anaphylaxis and has been associated with the development of Clostridial infections (gas gangrene). Cleansing with alcohol does not kill bacterial spores, and therefore, does not lower this risk.
Do not inject into digits, hands, or feet. Epinephrine is a strong vasoconstrictor. Accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area and has been associated with tissue necrosis.
5.2 Serious Infections at the Injection Site
Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported at the injection site following epinephrine injection for anaphylaxis. Clostridium spores can be present on the skin and introduced into the deep tissue with subcutaneous or intramuscular injection. While cleansing with alcohol may reduce presence of bacteria on the skin, alcohol cleansing does not kill Clostridium spores. To decrease the risk of Clostridium infection, do not inject Adrenalin ® into the buttock [see Warnings and Precautions (5.1)]. Advise patients to seek medical care if they develop signs or symptoms of infection, such as persistent redness, warmth, swelling, or tenderness, at the epinephrine injection site.
5.3 Disease Interactions
Some patients may be at greater risk for developing adverse reactions after systemic epinephrine administration. Despite these concerns, the presence of these conditions is not a contraindication to epinephrine administration in an acute, life-threatening situation.
Patients with Heart Disease
Epinephrine should be administered with caution in patients who have heart disease, including patients with cardiac arrhythmias, coronary artery or organic heart disease, cerebrovascular disease, or hypertension. In such patients, or in patients who are on drugs that may sensitize the heart to arrhythmias, epinephrine may precipitate or aggravate angina pectoris as well as produce ventricular arrhythmias [ see Drug Interactions (7) and Adverse Reactions (6)].
Other Patients and Diseases
Epinephrine should be administered with caution to patients with hyperthyroidism, Parkinson's disease, diabetes mellitus, pheochromocytoma, elderly individuals, and pregnant women. Patients with Parkinson's disease may experience psychomotor agitation or notice a temporary worsening of symptoms. Diabetic patients may experience transient increases in blood sugar.
5.4 Allergic Reactions Associated with Sulfite
Adrenalin ® contains sodium bisulfite which may cause mild to severe allergic reactions including anaphylaxis or asthmatic episodes in susceptible individuals. However, the presence of bisulfite in this product should not preclude its use for the treatment of serious allergic or other emergency situations even if the patient is sulfite-sensitive, as the alternatives to using epinephrine in a life-threatening situation may not be satisfactory.
-
6 ADVERSE REACTIONS
Common adverse reactions to systemically administered epinephrine include anxiety, apprehensiveness, restlessness, tremor, weakness, dizziness, sweating, palpitations, pallor, nausea and vomiting, headache, and respiratory difficulties. These symptoms occur in some persons receiving therapeutic doses of epinephrine, but are more likely to occur in patients with heart disease, hypertension, or hyperthyroidism [ see Warnings and Precautions (5.3)].
Due to the lack of randomized, controlled clinical trials of epinephrine for the treatment of anaphylaxis, the true incidence of adverse reactions associated with the systemic use of epinephrine is difficult to determine. Adverse reactions reported in observational trials, case reports, and studies are listed below by body system:
Cardiovascular: angina, arrhythmias, hypertension, pallor, palpitations, tachyarrhythmia, tachycardia, vasoconstriction, ventricular ectopy and stress cardiomyopathy.
Angina may occur in patients with coronary artery disease [ see Warnings and Precautions (5.3)].
Arrhythmias, including fatal ventricular fibrillation, have occurred, particularly in patients with underlying organic heart disease or patients receiving drugs that sensitize the heart to arrhythmias [ see Warnings and Precautions (5.3)].
Rapid rises in blood pressure associated with epinephrine use have produced cerebral hemorrhage, particularly in elderly patients with cardiovascular disease [ see Warnings and Precautions (5.3)].
Respiratory: respiratory difficulties.
Neurological: dizziness , disorientation , excitability , headache , impaired memory , lightheadedness , nervousness , panic, psychomotor agitation, sleepiness , tingling , tremor, and weakness.
Psychiatric: anxiety, apprehensiveness, restlessness.
Gastrointestinal: nausea, vomiting.
Other:
Patients with Parkinson's disease may experience psychomotor agitation or a temporary worsening of symptoms [ see Warnings and Precautions (5.3)].
Diabetic patients may experience transient increases in blood sugar [ see Warnings and Precautions (5.3)].
Accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area [ see Warnings and Precautions (5.1)]. Adverse events experienced as a result of an injection into these areas include increased heart rate, local reactions including injection site pallor, coldness, hypoesthesia, and tissue loss, or injury at the injection site resulting in bruising, bleeding, discoloration, erythema, and skeletal injury.
Injection into the buttock has resulted in cases of gas gangrene [ see Warnings and Precautions (5.1)].
Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported following epinephrine injection in the thigh [see Warnings and Precautions (5.2)] .
Skin: sweating.
To report SUSPECTED ADVERSE REACTIONS, contact Par Pharmaceutical at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
7 DRUG INTERACTIONS
Epinephrine should be administered cautiously to patients taking other sympathomimetic agents because of the possibility of additive effects.
Patients who are concomitantly receiving cardiac glycosides, digitalis, diuretics, quinidine, and other antiarrhythmics should be observed carefully for the development of cardiac arrhythmias [ see Warnings and Precautions (5.3) and Adverse Reactions (6)].
Administer epinephrine cautiously to patients receiving halogenated hydrocarbon general anesthetics, such as halothane, as coadministration may result in arrhythmias.
The effects of epinephrine may be potentiated by tricyclic antidepressants such as imipramine, monoamine oxidase inhibitors (MAOI), levothyroxine sodium, and certain antihistamines, notably diphenhydramine, tripelannamine, and dexchlorpheniramine.
The cardiostimulating and bronchodilating effects of epinephrine are antagonized by beta-adrenergic blocking drugs, such as propranolol.
The vasoconstricting and hypertensive effects of epinephrine are antagonized by alpha-adrenergic blocking drugs, such as phentolamine.
Ergot alkaloids may reverse the pressor effects of epinephrine.
Epinephrine should not be used to counteract circulatory collapse or hypotension caused by phenothiazines, as a reversal of the pressor effects of epinephrine may result in further lowering of blood pressure.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C.
There are no adequate and well-controlled studies in pregnant women. Epinephrine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus (fetal anoxia, spontaneous abortion, or both). Epinephrine is teratogenic in rabbits, mice and hamsters dosed during organogenesis.
Epinephrine has been shown to have teratogenic effects (including gastroschisis and embryonic lethality) when administered subcutaneous in rabbits at approximately 15 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m 2 basis at a maternal subcutaneous dose of 1.2 mg/kg/day for two to three days).
In mice, teratogenic effects (including embryonic lethality) were observed at approximately 3 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m 2 basis at maternal subcutaneous dose of 1 mg/kg/day for 10 days). These effects were not seen in mice at approximately 2 times the maximum recommended daily intramuscular or subcutaneous dose (on a mg/m 2 basis at a subcutaneous maternal dose of 0.5 mg/kg/day for 10 days).
In hamsters, teratogenic effects were observed at approximately 2 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m 2 basis at a maternal subcutaneous dose of 0.5 mg/kg/day for 4 days).
8.2 Labor and Delivery
Use with caution during labor and delivery. Although epinephrine improves maternal hypotension associated with anaphylaxis, it may result in uterine vasoconstriction, decreased uterine blood flow, and fetal anoxia.
8.3 Nursing Mothers
It is not known whether epinephrine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when epinephrine is administered to a nursing woman.
8.4 Pediatric Use
Clinical use data support weight-based dosing for treatment of anaphylaxis in pediatric patients, and other reported clinical experience with the use of epinephrine suggests that the adverse reactions seen in children are similar in nature and extent to those both expected and reported in adults.
8.5 Geriatric Use
Clinical studies for the treatment of anaphylaxis have not been performed in subjects aged 65 and over to determine whether they respond differently from younger subjects. However, other reported clinical experience with use of epinephrine for the treatment of anaphylaxis has identified that geriatric patients may be particularly sensitive to the effects of epinephrine. Therefore, for the treatment of anaphylaxis, consider starting with a lower dose to take into account potential concomitant disease or other drug therapy.
-
10 OVERDOSAGE
Overdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also result in pulmonary edema because of peripheral vascular constriction together with cardiac stimulation. Treatment consists of a rapidly acting α-adrenergic blocking drug and respiratory support.
Epinephrine is rapidly inactivated in the body and treatment following overdose with epinephrine is primarily supportive. If necessary, pressor effects may be counteracted by rapidly acting vasodilators or α-adrenergic blocking drugs. If prolonged hypotension follows such measures, it may be necessary to administer another pressor drug.
Epinephrine overdosage can also cause transient bradycardia followed by tachycardia and these may be accompanied by potentially fatal cardiac arrhythmias. Premature ventricular contractions may appear within one minute after injection and may be followed by multifocal ventricular tachycardia (prefibrillation rhythm). Subsidence of the ventricular effects may be followed by atrial tachycardia and occasionally by atrioventricular block. Treatment of arrhythmias consists of administration of a beta-adrenergic blocking drug such as propranolol.
Overdosage sometimes results in extreme pallor and coldness of the skin, metabolic acidosis due to elevated blood lactic acid levels, and kidney failure. Suitable corrective measures must be taken in such situations.
Myocardial ischemia, myocardial infarction and cardiomyopathy have been noted in the literature following overdose of epinephrine.
-
11 DESCRIPTION
Adrenalin ® (epinephrine injection, USP) is a clear, colorless, sterile solution containing 1 mg/mL epinephrine, packaged as 1 mL of solution in a single-use clear glass vial or 30 mL of solution in a multiple-dose amber glass vial. In the 1 mL vial, each 1 mL of Adrenalin ® solution contains 1 mg epinephrine, 7.3 mg sodium chloride, 0.457 mg sodium metabisulfite, 1 mg sodium hydroxide, 2.25 mg tartaric acid, 0.20 mg disodium edetate dihydrate, hydrochloric acid to adjust pH, and water for injection. In the 30 mL vial, each 1 mL of Adrenalin ® solution contains 1 mg epinephrine, 6.15 mg sodium chloride, 0.457 mg sodium metabisulfite, 0.920 mg sodium hydroxide, 2.25 mg tartaric acid, 0.20 mg disodium edetate dihydrate, hydrochloric acid to adjust pH, 5.25 mg chlorobutanol as a preservative and water for injection. The pH range is 2.2-5.0.
Epinephrine is a sympathomimetic catecholamine. The chemical name of epinephrine is: 1,2-Benzenediol, 4-[(1R)-1-hydroxy-2-(methylamino)ethyl]-, or (-)-3,4-Dihydroxy-α-[2-(methylamino)ethyl]benzyl alcohol.
The chemical structure of epinephrine is:
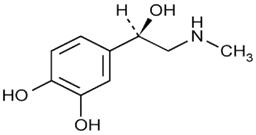
The molecular weight of epinephrine is 183.2.
Epinephrine solution deteriorates rapidly on exposure to air or light, turning pink from oxidation to adrenochrome and brown from the formation of melanin.
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Through its action on alpha-adrenergic receptors, epinephrine lessens the vasodilation and increased vascular permeability that occurs during anaphylaxis, which can lead to loss of intravascular fluid volume and hypotension.
Through its action on beta-adrenergic receptors, epinephrine causes bronchial smooth muscle relaxation and helps alleviate bronchospasm, wheezing and dyspnea that may occur during anaphylaxis.
Epinephrine also alleviates pruritus, urticaria, and angioedema and may relieve gastrointestinal and genitourinary symptoms associated with anaphylaxis because of its relaxer effects on the smooth muscle of the stomach, intestine, uterus and urinary bladder.
Epinephrine increases glycogenolysis, reduces glucose up take by tissues, and inhibits insulin release in the pancreas, resulting in hyperglycemia and increased blood lactic acid [ see Warnings and Precautions (5.3)].
Epinephrine causes mydriasis when administered parenterally.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long- term studies to evaluate the carcinogenic potential of epinephrine have not been conducted.
Epinephrine and other catecholamines have been shown to have mutagenic potential in vitro. Epinephrine was positive in the Salmonella bacterial reverse mutation assay, positive in the mouse lymphoma assay, and negative in the in vivo micronucleus assay. Epinephrine is an oxidative mutagen based on the E. coli WP2 Mutoxitest bacterial reverse mutation assay. This should not prevent the use of epinephrine under the conditions noted under Indications and Usage (1).
The potential for epinephrine to impair reproductive performance has not been evaluated, but epinephrine has been shown to decrease implantation in female rabbits dosed subcutaneously with 1.2 mg/kg/day (15-fold the highest human intramuscular or subcutaneous daily dose) during gestation days 3 to 9.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Adrenalin ® 1 mL Single-Use Vials:
Each carton contains 25 single-use vials containing 1 mL Adrenalin ® (epinephrine injection, USP) solution 1 mg/mL in a 3 mL clear glass vial.
NDC 42023-159-25 1 mL vial
Adrenalin ® 30 mL Multi-Dose Vials:
Each carton contains 1 multiple-dose vial containing 30 mL Adrenalin ® (epinephrine injection, USP) solution 1 mg/mL in a 36 mL amber glass vial.
NDC 42023-168-01 30 mL vial
Vial and contents must be discarded 30 days after initial use.
Store between 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Epinephrine is light sensitive. Protect from light and freezing.
Inspect visually for particulate matter and discoloration prior to administration. Do not use the solution if it is colored or cloudy, or if it contains particulate matter.
-
17 PATIENT COUNSELING INFORMATION
Advise patients or their caregivers about common adverse reactions associated with the use of epinephrine including an increase in heart rate, the sensation of a more forceful heartbeat, palpitations, sweating, nausea and vomiting, difficulty breathing, pallor, dizziness, weakness or shakiness, headache, apprehension, nervousness, or anxiety. These symptoms and signs usually subside rapidly, especially with rest, quiet and recumbent positioning.
Warn patients with a good response to initial treatment about the possibility of recurrence of symptoms and instruct patients to obtain proper medical attention if symptoms return.
Warn patients with diabetes that they may develop increased blood glucose levels following epinephrine administration.
Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported at the injection site following epinephrine injection for anaphylaxis. Advise patients to seek medical care if they develop signs or symptoms of infection, such as persistent redness, warmth, swelling, or tenderness, at the epinephrine injection site [see Warnings and Precautions (5.2)].
- SPL UNCLASSIFIED SECTION
-
INSTRUCTIONS FOR USE
ALCOHOL PREP PAD- isopropyl alcohol swab
Drug Facts
Active ingredientIsopropyl Alcohol 70% v/v
PurposeFirst Aid Antiseptic
UseFor preparation of the skin prior to an injection
Warnings- For external use only
- Flammable, keep away from fire or flame
Do Not Use
- Do not use with electrocautery procedures
- Do not use in the eyes
- Do not apply to irritated skin
- Stop use if pain, irritation, redness, or swelling occurs, discontinue use and consult a physician.
- Keep out from reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Open packet
- Remove pad
- Apply topically as needed to cleanse intended area. Discard after single use.
Other information
- Store at room temperature 59-86°F (15-30°C)
- Contents sterile in unopened, undamaged packag
Inactive ingredients
purified water
-
Instructions for Use
EpinephrineSnap™-V
Epinephrine convenience kit
Rx Only
NDC 71923-200-20
Single use only
For intramuscular or subcutaneous use
Not for intravenous or ophthalmic use
Indicated for patients weighing greater than or equal to 15 kg (33 lbs)
Keep out of reach of children
Store at 20 to 25 degrees C (68 to 77 degrees F)
Indications and Use
The epinephrine in this kit is a non-selective alpha and beta adrenergic agonist indicated for the emergency treatment of allergic reactions, including anaphylaxis.
Dosage and Administration
Always follow the prescriber’s order for dosing and administration. Epinephrine may be administered for anaphylaxis:
Adults and Children 30 kg (66 lbs.) or more 0.3 to 0.5 mg (0.3 to 0.5 mL) of undiluted epinephrine administered intramuscularly or subcutaneously in the anterolateral aspect of the thigh, up to a maximum of 0.5 mg (0.5 mL) per injection, repeated every 5 to 10 minutes as necessary. Monitor clinically for reaction severity and cardiac effects.
Children 15-30 kg (33-66 lbs.) 0.01mg/kg (0.01 mL/ kg) of undiluted epinephrine administered intramuscularly or subcutaneously in the anterolateral aspect of the thigh, up to a maximum of 0.3 mg (0.3 mL) every 5 to 10 minutes as necessary. Maximum single dose is 0.3 mg (0.3 mL) per injection, repeated every 5 to 10 minutes as necessary. Monitor clinically for reaction severity and cardiac effects.
The Epinephrine Syringe does not have markings for doses less than 0.15 mL, or the equivalent of a dose of 0.15 mg.
Monitor patient clinically for the severity of the allergic reaction and the potential cardiac effects of the drug, with repeat doses titrated as necessary. *
See epinephrine full prescribing information at parsterileproducts.com
Product Information
EpinephrineSnap™-V is an epinephrine convenience kit designed for use by licensed health care professionals authorized to administer prescription medication.
Each kit is designed to treat a single episode of an allergic reaction/ anaphylaxis. The epinephrine provided in this kit must be used on the patient experiencing signs and symptoms of an allergic reaction/ anaphylaxis or discarded in an appropriate sharps container within 30 minutes of entering the epinephrine vial. Prepared syringes may not be stored for later use.
Each EpinephrineSnap™- V kit contains
(1) Vial of epinephrine injection 1:1000 1 mg/ mL for intramuscular or subcutaneous use
(1) Epinephrine package insert
(4) Alcohol Prep Pads
(3) 23-gauge 1-inch safety needles
(3) 1 mL luer lock Epinephrine Syringes™ with markings of 0.15 mL, 0.3 mL and 0.5 mL.
(1) Instructions For Use card
Epinephrine is light sensitive; protect from light and freezing.
See epinephrine package insert for INDICATIONS, and USAGE, CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, ADVERSE REACTIONS and OVERDOSAGE.
Instructions for Use
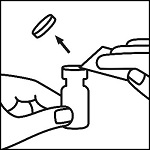
Remove inner tray from outer packaging, place on a flat surface. Remove the epinephrine vial. Inspect the epinephrine vial for particulate matter and discoloration prior to administration. Do not use if the solution is colored or cloudy or if it contains particulate matter. Snap off the lid of the Adrenalin ® vial and wipe the rubber stopper with an alcohol prep pad.
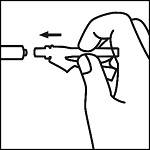
Remove the safety needles and The Epinephrine Syringes™. Attach a safety needle to The Epinephrine Syringe™ and insert needle through the rubber stopper of the epinephrine vial, drawing out a single dose.
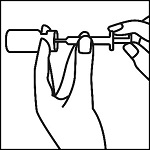
Always follow the prescriber’s order for dosing information.
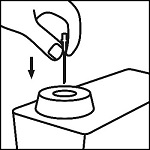
Prepare to administer the subcutaneous or intramuscular injection. The preferred site of epinephrine administration is the anterolateral aspect of the thigh (vastus lateralis muscle) because of its location, size and available blood flow. This location is between the greater trochanter and the knee. When injecting, lift the vastus lateralis muscle away from the bone. Cleanse the site of administration with an alcohol pad prior to injection, however, epinephrine may be injected through clothing if necessary.
Do not inject into the buttocks, digits, hands or feet.
If the patient’s symptoms of anaphylaxis persist, epinephrine dosing may be repeated every 5- 10 minutes per the prescriber’s order.
Do not administer repeated injections of epinephrine in the same site as the resulting vasoconstriction will cause tissue necrosis.
Any unused epinephrine either in the vial or syringes must be discarded 30 minutes after entering the vial.
*parsterileproducts.com/products/asset s/pdf/PI/2018/Adrenalin-PI-2018.pdf
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit fda.gov/ Safety/MedWatch/ or call 1-800-FDA-1088
For more information, go to epinephrinesnap.com
Snap Medical Industries
545 Metro Place South Suite 100
Dublin, Ohio 43017
Phone: 1-800-875-4508
Adrenalin ® is a registered trademark of Par Sterile Products, LLC (Chestnut Ridge, NY).
EpinephrineSnap™-V IFU | 06/2019
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – Carton Label
NDC 71923-100-20
Rx Only
For use by licensed health care professionals authorized to administer prescription medication
Keep out of reach of children
For intramuscular or subcutaneous administration
Not for intravenous or ophthalmic use
Indicated for patients weighing greater than or equal to 15 kg (33 lbs)
Single use only
Epinephrine is light sensitive; protect from light and freezing
Kit contains
epinephrine injection
1 mg/mL (1:1000) in a 1 mL single-use vial and supplies for administration
Epinephrine 1 mg/mLepinephrinesnap TM-V
EPINEPHRINE CONVENIENCE KIT
-
INGREDIENTS AND APPEARANCE
EPINEPHRINESNAP-V
epinephrine convenience kit kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71923-200 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71923-200-20 1 in 1 CARTON; Type 0: Not a Combination Product 12/01/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1 mL Part 2 1 PACKET 1 mL Part 1 of 2 ADRENALIN
epinephrine injectionProduct Information Item Code (Source) NDC:42023-159 Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength TARTARIC ACID (UNII: W4888I119H) 2.25 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM CHLORIDE (UNII: 451W47IQ8X) 7.3 mg in 1 mL EDETATE DISODIUM (UNII: 7FLD91C86K) 0.2 mg in 1 mL WATER (UNII: 059QF0KO0R) SODIUM METABISULFITE (UNII: 4VON5FNS3C) 0.457 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) 1 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42023-159-25 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204200 07/01/2013 Part 2 of 2 MCKESSON ALCOHOL PREP PAD
isopropyl alcohol swabProduct Information Item Code (Source) NDC:68599-5804 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68599-5804-1 1 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 04/09/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204200 12/01/2018 Labeler - Snap Medical Industries (036735821) Establishment Name Address ID/FEI Business Operations Snap Medical Industries, LLC 036735821 repack(71923-200) , manufacture(71923-200) Establishment Name Address ID/FEI Business Operations Par Sterile Products LLC 808402890 manufacture(42023-159) Establishment Name Address ID/FEI Business Operations Carton Service, Incorporated 928861723 label(71923-200) , pack(71923-200) , relabel(71923-200) , repack(71923-200) Establishment Name Address ID/FEI Business Operations Woodfield Distribution, LLC 962363888 relabel(71923-200) , repack(71923-200)