VETRASEB SSB- benzoyl peroxide, sodium thiosulfate, salicylic acid, phytosphingosine hydrochloride shampoo
MWI/VetOne
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
VetraSeb™ SSB Shampoo
ACTIVE INGREDIENTS: Benzoyl Peroxide 3%, Salicylic Acid 2%, Sodium Thiosulfate 2%, Phytosphingosine HCI 0.05%.
INACTIVE INGREDIENTS: Water, Sodium C14-16 Olefin Sulfonate, Propylene Glycol, Acrylates Copolymer, Myristamine Oxide, Fragrance, Tocopheryl Acetate, Sodium Dioctyl Sulfosuccinate, Methylchloroisothiazolinone, Methylisothiazolinone.
PROPERTIES: VetraSeb™ SSB Shampoo is a cleansing and degreasing, soap-free shampoo for the relief of scaling and itching associated with skin infections, seborrheic disorders, and follicular plugging on dogs and cats. The combination of benzoyl peroxide, micronized sulfur and salicylic acid allows for degreasing, follicular flushing, and enhanced keratolytic and keratoplastic activity. VetraSeb™ SSB Shampoo contains phytosphingosine, a pro-ceramide to help support a healthy skin barrier. Actives in this shampoo also have antimicrobial properties.
INDICATION: For use on dogs and cats only. For the management of skin infections and seborrheic disorders.
DIRECTIONS FOR USE: Wet the animal with warm water and apply VetraSeb™ SSB Shampoo. Massage into the wet hair coat. Allow to remain on the hair coat for 5 to 10 minutes before rinsing thoroughly with clean water. Repeat if needed. Apply 3 times per week or as directed by your veterinarian. May be used as part of a management plan as recommended by your veterinarian.
CAUTIONS: For external use on dogs and cats only. Avoid contact with the eyes. In case of contact with eyes, rinse thoroughly and consult your veterinarian. If skin irritation occurs or increases, discontinue use and consult with your veterinarian.
WARNING: Keep out of the reach of children.
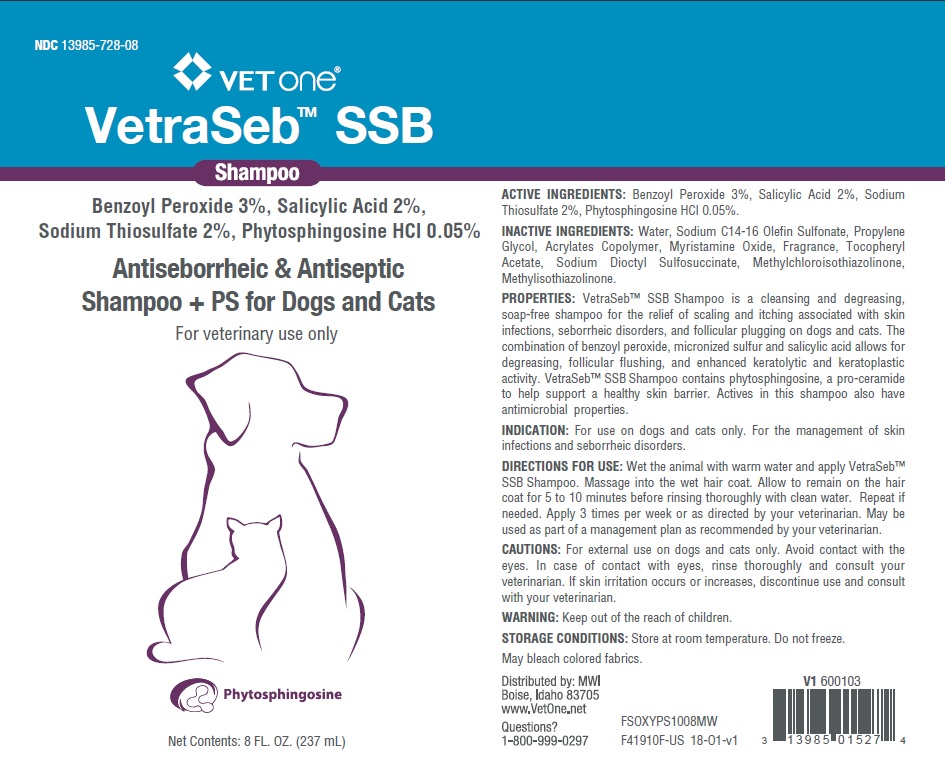
PRINCIPAL DISPLAY PANEL - 8 OUNCE (237 mL) Bottle
NDC 13985-728-08
VETone®
VetraSeb™ SSB
Shampoo
Benzoyl Peroxide 3%, Salicylic Acid 2%,
Sodium Thiosulfate 2%, Phytosphingosine HCl 0.05%
Antiseborrheic and Antiseptic
Shampoo +PS for Cats and Dogs
For veterinary use only
Phytosphingosine
8 FL. OZ. (237 mL)
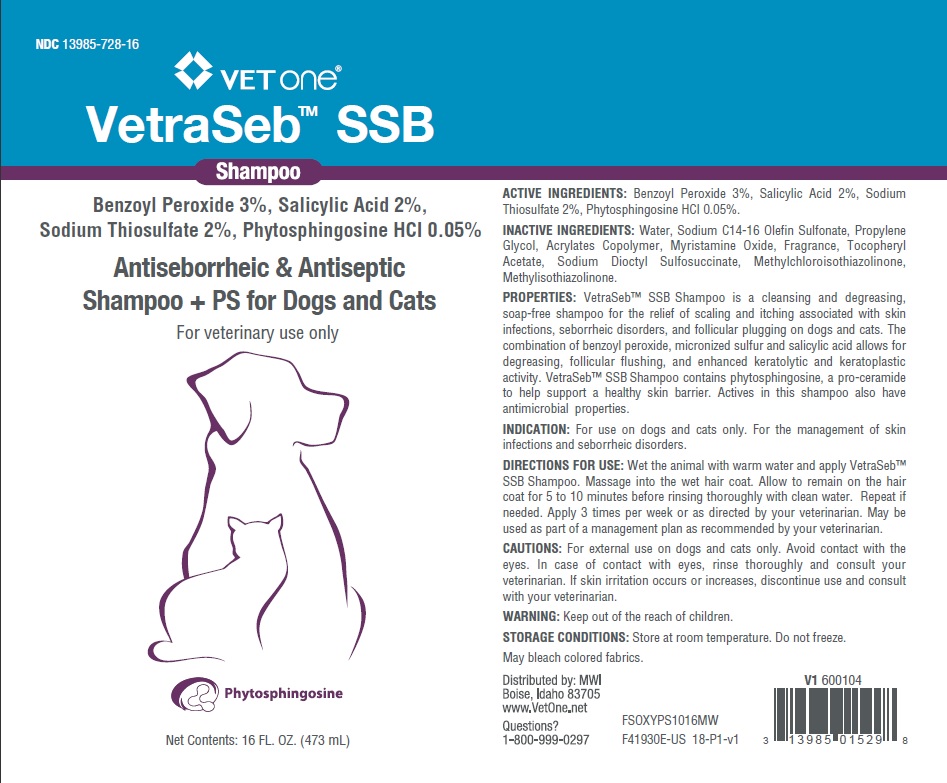
PRINCIPAL DISPLAY PANEL - 16 OUNCE (473 mL) Bottle
NDC 13985-728-16
VETone®
VetraSeb™ SSB
Shampoo
Benzoyl Peroxide 3%, Salicylic Acid 2%,
Sodium Thiosulfate 2%, Phytosphingosine HCl 0.05%
Antiseborrheic and Antiseptic
Shampoo +PS for Cats and Dogs
For veterinary use only
Phytosphingosine
16 FL. OZ. (473 mL)
| VETRASEB
SSB
benzoyl peroxide, sodium thiosulfate, salicylic acid, phytosphingosine hydrochloride shampoo |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - MWI/VetOne (019926120) |
| Registrant - Ceva Sante Animale (261126049) |