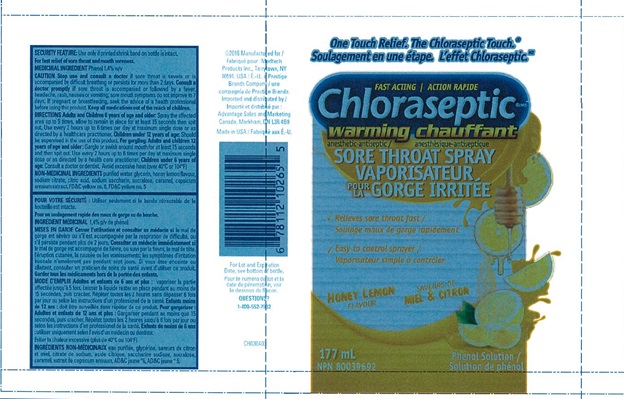
CHLORASEPTIC WARMING SORE THROAT- phenol spray
Denison Pharmaceuticals, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Chloraseptic Warming Sore Throat Spray
Warnings
Sore Throat Warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a doctor promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by a doctor.
Directions
-
Adults and children 6 years of age and older:
- Apply to the affected area (one spray).
- Allow to remain in place for at least 15 seconds, then spit out.
- Use every 2 hours or as directed by a doctor or dentist.
Children under 12 years of age should be supervised in the use of this product.
Children under 3 6 years of age: consult a doctor or dentist.
Other information
- Store at room temperature.
- Check expiration date before using.
- Contains FD&C yellow 5 (tartrazine) as a color additive
| CHLORASEPTIC WARMING SORE THROAT
phenol spray |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Denison Pharmaceuticals, Inc. (001207208) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Denison Pharmaceuticals, Inc. | 001207208 | MANUFACTURE(0295-1026) | |
Revised: 5/2017
Document Id: f49b6e1e-e825-4936-95cc-15df268dc2a9
Set id: 782759c2-4755-411f-bb4d-aa9a1b3db442
Version: 2
Effective Time: 20170503
Denison Pharmaceuticals, Inc.