Label: NUMFAST TETRACAINE GREEN- tetracaine cream
- NDC Code(s): 67194-020-01
- Packager: Unit Dose, Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
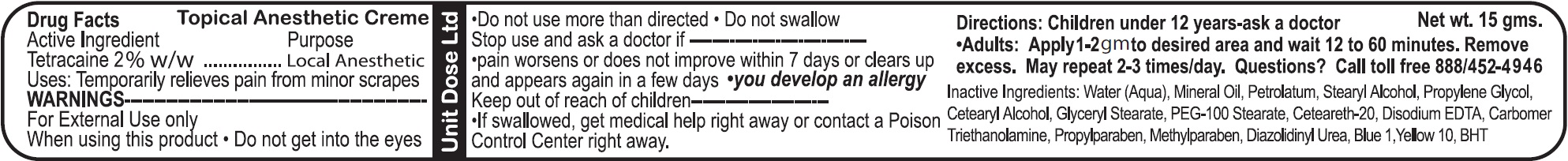
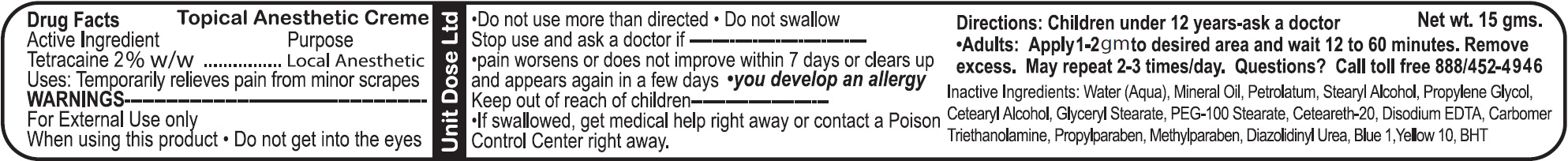
- Drug Facts
- Active Ingredient
- Uses:
- WARNINGS
- Directions:
- Questions?
- Inactive Ingredients:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
NUMFAST TETRACAINE GREEN
tetracaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67194-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRACAINE (UNII: 0619F35CGV) (TETRACAINE - UNII:0619F35CGV) TETRACAINE 20 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) EDETATE DISODIUM (UNII: 7FLD91C86K) TROLAMINE (UNII: 9O3K93S3TK) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67194-020-01 15 g in 1 JAR; Type 0: Not a Combination Product 01/20/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/20/2016 Labeler - Unit Dose, Ltd. (119080393)