Label: MESALAMINE tablet, delayed release
- NDC Code(s): 60687-397-25, 60687-397-95
- Packager: American Health Packaging
- This is a repackaged label.
- Source NDC Code(s): 68382-711
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated December 14, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MESALAMINE DELAYED-RELEASE TABLETS safely and effectively. See full prescribing information for MESALAMINE DELAYED-RELEASE TABLETS.
MESALAMINE Delayed-Release Tablets, for oral use
Initial U.S. Approval: 1987RECENT MAJOR CHANGES
- Warnings and Precautions, Renal Impairment (5.1) 11/2022
INDICATIONS AND USAGE
Mesalamine delayed-release tablets are an aminosalicylate indicated for the
- induction and maintenance of remission in adult patients with mildly to moderately active ulcerative colitis. (1)
DOSAGE AND ADMINISTRATION
Administration Instructions
- Evaluate renal function prior to initiation of mesalamine and periodically while on therapy. (2, 5.1)
- Swallow mesalamine delayed-release tablets whole; do not split or crush. (2)
- Administer mesalamine delayed-release tablets with food. (2)
- Drink an adequate amount of fluids. (2, 5.8)
Recommended Dosage in Adults
DOSAGE FORMS AND STRENGTHS
Delayed-Release Tablets: 1.2 g (3)
CONTRAINDICATIONS
Known or suspected hypersensitivity to salicylates or aminosalicylates or to any of the ingredients of mesalamine delayed-release tablets. (4)
WARNINGS AND PRECAUTIONS
- Renal Impairment: Assess renal function at the beginning of treatment and periodically during treatment. Evaluate the risks and benefits of mesalamine in patients with known renal impairment or taking nephrotoxic drugs. (5.1, 7.1, 8.6)
- Mesalamine-Induced Acute Intolerance Syndrome: Symptoms may be difficult to distinguish from an ulcerative colitis exacerbation. Monitor for worsening symptoms while on treatment. Discontinue treatment if acute intolerance syndrome is suspected. (5.2)
- Hypersensitivity Reactions, including myocarditis and pericarditis: Evaluate patients immediately and discontinue mesalamine if a hypersensitivity reaction is suspected. (5.3)
- Hepatic Failure: Evaluate the risks and benefits of using mesalamine in patients with known liver impairment. (5.4)
- Severe Cutaneous Adverse Reactions: Discontinue at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation. (5.5)
- Upper Gastrointestinal Tract Obstruction: Avoid in patients with pyloric stenosis or other organic or functional obstruction. (5.6)
- Photosensitivity: Advise patients with pre-existing skin conditions to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors. (5.7)
- Nephrolithiasis: Cases of nephrolithiasis have been reported with the use of mesalamine. Mesalamine-containing stones are undetectable by standard radiography or computed tomography (CT). Ensure adequate hydration during treatment. (5.8)
- Interference With Laboratory Tests: Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection. (5.9)
ADVERSE REACTIONS
Most common adverse reactions in:
- adults (≥2%) are headache, flatulence, liver function test abnormal, abdominal pain, and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals (USA) Inc. at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Geriatric Patients: Increased risk of blood dyscrasias; monitor complete blood cell counts and platelet counts. (8.5)
Pediatric use information is approved for Takeda Pharmaceuticals U.S.A., Inc.’s LIALDA (mesalamine) delayed-release tablets. However, due to Takeda Pharmaceuticals U.S.A., Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
5.2 Mesalamine-Induced Acute Intolerance Syndrome
5.3 Hypersensitivity Reactions
5.4 Hepatic Failure
5.5 Severe Cutaneous Adverse Reactions
5.6 Upper Gastrointestinal Tract Obstruction
5.7 Photosensitivity
5.8 Nephrolithiasis
5.9 Interference with Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
7.2 Azathioprine and 6-Mercaptopurine
7.3 Interference with Urinary Normetanephrine Measurements
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Adults with Mildly to Moderately Active Ulcerative Colitis
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Mesalamine delayed-release tablets are indicated for the
- induction and maintenance of remission in adult patients with mildly to moderately active ulcerative colitis.
Pediatric use information is approved for Takeda Pharmaceuticals U.S.A., Inc.’s LIALDA (mesalamine) delayed-release tablets. However, due to Takeda Pharmaceuticals U.S.A., Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
-
2 DOSAGE AND ADMINISTRATION
Administration Instructions
- Evaluate renal function prior to initiation of mesalamine and periodically while on therapy.
- Swallow mesalamine delayed-release tablets whole; do not split or crush.
- Administer mesalamine delayed-release tablets with food [see Clinical Pharmacology (12.3)].
- Drink an adequate amount of fluids [see Warnings and Precautions (5.8)].
Adults
- The recommended dosage for the induction of remission in adult patients with mildly to moderately active ulcerative colitis is 2.4 g to 4.8 g (two to four 1.2-g tablets) taken once daily.
- The recommended dosage for the maintenance of remission is 2.4 g (two 1.2-g tablets) taken once daily.
Pediatric use information is approved for Takeda Pharmaceuticals U.S.A., Inc.’s LIALDA (mesalamine) delayed-release tablets. However, due to Takeda Pharmaceuticals U.S.A., Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Mesalamine delayed-release tablets are contraindicated in patients with known or suspected hypersensitivity to salicylates, aminosalicylates, or to any of the ingredients of mesalamine delayed-release tablets [see Warnings and Precautions (5.3), Adverse Reactions (6.2), Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
Renal impairment, including minimal change disease, acute and chronic interstitial nephritis, and, rarely, renal failure, has been reported in patients given products such as mesalamine delayed-release tablets that contain mesalamine or are converted to mesalamine. In animal studies, the kidney was the principal organ of mesalamine toxicity [see Adverse Reactions (6.2), Nonclinical Toxicology (13.2)].
Evaluate renal function prior to initiation of mesalamine therapy and periodically while on therapy. Evaluate the risks and benefits of using mesalamine in patients with known renal impairment, history of renal disease, or taking concomitant nephrotoxic drugs. Discontinue mesalamine if renal function deteriorates while on therapy [see Drug Interactions (7.1), Use in Specific Populations (8.6)].
5.2 Mesalamine-Induced Acute Intolerance Syndrome
Mesalamine has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. Although the exact frequency of occurrence has not been determined, it has occurred in 3% of patients in controlled clinical trials of mesalamine or sulfasalazine. Symptoms include cramping, acute abdominal pain and bloody diarrhea, and sometimes fever, headache, and rash. Monitor patients closely for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with mesalamine delayed-release tablets.
5.3 Hypersensitivity Reactions
Hypersensitivity reactions have been reported in patients taking sulfasalazine. Some of these patients may have a similar reaction to mesalamine delayed-release tablets or to other compounds that contain or are converted to mesalamine.
As with sulfasalazine, mesalamine-induced hypersensitivity reactions may present as internal organ involvement, including myocarditis, pericarditis, nephritis, hepatitis, pneumonitis, and hematologic abnormalities. Evaluate patients immediately if signs or symptoms of a hypersensitivity reaction are present. Discontinue mesalamine delayed-release tablets if an alternative etiology for the signs or symptoms cannot be established.
5.4 Hepatic Failure
There have been reports of hepatic failure in patients with pre-existing liver disease who have been administered mesalamine. Evaluate the risks and benefits of using mesalamine in patients with known liver impairment.
5.5 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported with the use of mesalamine [see Adverse Reactions (6.2)]. Discontinue mesalamine delayed-release tablets at the first appearance of signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation.
5.6 Upper Gastrointestinal Tract Obstruction
Pyloric stenosis or other organic or functional obstruction in the upper gastrointestinal tract may cause prolonged gastric retention of mesalamine delayed-release tablets, which would delay mesalamine release in the colon. Avoid mesalamine in patients at risk of upper gastrointestinal tract obstruction.
5.7 Photosensitivity
Patients with pre-existing skin conditions such as atopic dermatitis and atopic eczema have reported more severe photosensitivity reactions. Advise patients to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors.
5.8 Nephrolithiasis
Cases of nephrolithiasis have been reported with the use of mesalamine, including stones with a 100% mesalamine content. Mesalamine-containing stones are radiotransparent and undetectable by standard radiography or computed tomography (CT). Ensure adequate hydration during treatment with mesalamine delayed-release tablets.
5.9 Interference with Laboratory Tests
Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection because of the similarity in the chromatograms of normetanephrine and the main metabolite of mesalamine, N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA). Consider an alternative, selective assay for normetanephrine.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Renal impairment, including renal failure [see Warnings and Precautions (5.1)]
- Mesalamine-induced acute intolerance syndrome [see Warnings and Precautions (5.2)]
- Hypersensitivity reactions [see Warnings and Precautions (5.3)]
- Hepatic failure [see Warnings and Precautions (5.4)]
- Severe cutaneous adverse reactions [see Warnings and Precautions (5.5)]
- Upper gastrointestinal tract obstruction [see Warnings and Precautions (5.6)]
- Photosensitivity [see Warnings and Precautions (5.7)]
- Nephrolithiasis [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults
Induction
The most common adverse reactions occurring in at least 1% of mesalamine -or placebo-treated adult patients with mildly to moderately active ulcerative colitis in two eight-week, randomized, double-blind, placebo-controlled trials (Study 1 and Study 2) [see Clinical Studies (14.1)] are listed in Table 2.Table 2 Adverse Reactions *in Two Eight-Week, Placebo-Controlled Trials of Induction Therapy (Study 1 and Study 2) in Adults with Mildly to Moderately Active Ulcerative Colitis - *
- Reported in at least 1% of patients in at least one mesalamine group and greater than placebo.
Adverse Reaction
Mesalamine Delayed-Release Tablets 2.4 g once daily
(n = 177)
Mesalamine Delayed-Release Tablets 4.8 g once daily
(n = 179)
Placebo
(n = 179)
Headache
6%
3%
<1%
Flatulence
4%
3%
3%
Liver Function Test Abnormal
<1%
2%
1%
Alopecia
0
1%
0
Pruritus
<1%
1%
1%
Pancreatitis occurred in less than 1% of patients during induction in clinical trials and resulted in discontinuation of therapy with mesalamine in patients experiencing this event.
Maintenance of Remission
A mesalamine dosage of 2.4 g/day, administered as either 1.2 g twice daily or 2.4 g once daily, was evaluated for safety in three maintenance trials in patients with mildly to moderately active ulcerative colitis: a 6-month double-blind, active-controlled study (Study 3) [see Clinical Studies (14.1)] and two 12 to 14 month open-label studies. The most common adverse reactions with mesalamine in these maintenance trials are listed in Table 3.Table 3 Adverse Reactions *in Three Trials of Maintenance of Remission in Adults with Ulcerative Colitis Mesalamine Delayed-Release Tablets 2.4 g/day†
(n=1082)
Adverse Reaction
%
Headache
3%
Liver function test abnormal
2%
Abdominal pain
2%
Diarrhea
2%
Abdominal distension
1%
Abdominal pain upper
1%
Dyspepsia
1%
Back pain
1%
Rash
1%
Arthralgia
1%
Fatigue
1%
Hypertension
1%
The following adverse reactions, presented by body system, were reported in less than 1% of mesalamine delayed-release tablets-treated patients with ulcerative colitis in either induction or maintenance trials:
Cardiac Disorder
TachycardiaEar and Labyrinth Disorders
Ear painGastrointestinal Disorders
Abdominal distention, colitis, diarrhea, flatulence, nausea, pancreatitis, rectal polyp, vomitingGeneral Disorders and Administrative Site Disorders
Asthenia, face edema, fatigue, pyrexiaInvestigations
Decreased platelet countMusculoskeletal and Connective Tissue Disorders
Arthralgia, back painNervous System Disorders
Dizziness, somnolence, tremorRespiratory, Thoracic and Mediastinal Disorders
Pharyngolaryngeal painSkin and Subcutaneous Tissue Disorders
Acne, prurigo, rash, alopecia, pruritus, urticariaVascular Disorders
Hypertension, hypotensionPediatric use information is approved for Takeda Pharmaceuticals U.S.A., Inc.’s LIALDA (mesalamine) delayed-release tablets. However, due to Takeda Pharmaceuticals U.S.A., Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of mesalamine delayed-release tablets or other mesalamine-containing products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole
Lupus-like syndrome, drug feverCardiac Disorders
Pericarditis, pericardial effusion, myocarditis [see Warnings and Precautions (5.3)]Gastrointestinal
Cholecystitis, gastritis, gastroenteritis, gastrointestinal bleeding, perforated peptic ulcerHepatic
Jaundice, cholestatic jaundice, hepatitis, liver necrosis, liver failure, hepatotoxicity, Kawasaki-like syndrome including changes in liver enzymesHematologic
Agranulocytosis, aplastic anemiaImmune System Disorders
Anaphylactic reaction, angioedemaMusculoskeletal and Connective Tissue Disorders
Myalgia, lupus-like syndromeNeurological/Psychiatric
Peripheral neuropathy, Guillain-Barre syndrome, transverse myelitis, intracranial hypertensionRenal Disorders
Renal failure, interstitial nephritis, nephrogenic diabetes insipidus, nephrolithiasis [see Warnings and Precautions (5.1, 5.8)]Respiratory, Thoracic and Mediastinal Disorders
Interstitial lung disease, hypersensitivity pneumonitis (including interstitial pneumonitis, allergic alveolitis, eosinophilic pneumonitis), pleurisy/pleuritisSkin
Psoriasis, pyoderma gangrenosum, erythema nodosum, photosensitivity, SJS/TEN, DRESS, and AGEP [see Warnings and Precautions (5.5)]Urogenital
Reversible oligospermia -
7 DRUG INTERACTIONS
7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
The concurrent use of mesalamine with known nephrotoxic agents, including non-steroidal anti-inflammatory drugs (NSAIDs), may increase the risk of nephrotoxicity. Monitor patients taking nephrotoxic drugs for changes in renal function and mesalamine-related adverse reactions [see Warnings and Precautions (5.1)].
7.2 Azathioprine and 6-Mercaptopurine
The concurrent use of mesalamine with azathioprine or 6-mercaptopurine and/or any other drugs known to cause myelotoxicity may increase the risk for blood disorders, bone marrow failure, and associated complications. If concomitant use of mesalamine and azathioprine or 6-mercaptopurine cannot be avoided, monitor blood tests, including complete blood cell counts and platelet counts.
7.3 Interference with Urinary Normetanephrine Measurements
Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection [see Warnings and Precautions (5.9)]. Consider an alternative, selective assay for normetanephrine.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Published data from meta-analyses, cohort studies, and case series on the use of mesalamine during pregnancy have not reliably informed an association with mesalamine and major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). There are adverse effects on maternal and fetal outcomes associated with ulcerative colitis in pregnancy (see Clinical Considerations).In animal reproduction studies, there were no adverse developmental outcomes with administration of oral mesalamine during organogenesis to pregnant rats and rabbits at doses 1.8 and 2.9 times, respectively, the maximum recommended human dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo/fetal risk
Published data suggest that increased disease activity is associated with the risk of developing adverse pregnancy outcomes in women with ulcerative colitis. Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (less than 2500 g) infants, and small for gestational age at birth.Data
Human Data
Published data from meta-analyses, cohort studies, and case series on the use of mesalamine during early pregnancy (first trimester) and throughout pregnancy have not reliably informed an association of mesalamine and major birth defects, miscarriage, or adverse maternal or fetal outcomes. There is no clear evidence that mesalamine exposure in early pregnancy is associated with an increased risk of major congenital malformations, including cardiac malformations. Published epidemiologic studies have important methodological limitations which hinder interpretation of the data, including inability to control for confounders, such as underlying maternal disease, maternal use of concomitant medications, and missing information on the dose and duration of use for mesalamine products.Animal Data
Reproduction studies with mesalamine during organogenesis have been performed in rats at doses up to 1000 mg/kg/day (1.8 times the maximum recommended human dose based on a body surface area comparison) and rabbits at doses up to 800 mg/kg/day (2.9 times the maximum recommended human dose based on a body surface area comparison) and have revealed no evidence of harm to the fetus due to mesalamine.8.2 Lactation
Risk Summary
Data from published literature report the presence of mesalamine and its metabolite, N-acetyl-5-aminosalicylic acid in human milk in small amounts with relative infant doses (RID) of 0.1% or less for mesalamine (see Data). There are case reports of diarrhea in breastfed infants exposed to mesalamine (see Clinical Considerations). There is no information on the effects of the drug on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of mesalamine to an infant during lactation; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for mesalamine and any potential adverse effects on the breastfed child from mesalamine or from the underlying maternal condition.Clinical Considerations
Advise the caregiver to monitor the breastfed infant for diarrhea.Data
In published lactation studies, maternal mesalamine doses from various oral and rectal formulations and products ranged from 500 mg to 4.8 g daily. The average concentration of mesalamine in milk ranged from non-detectable to 0.5 mg/L. The average concentration of N-acetyl-5-aminosalicylic acid in milk ranged from 0.2 to 9.3 mg/L. Based on these concentrations, estimated infant daily dosages for an exclusively breastfed infant are 0 to 0.075 mg/kg/day (RID 0% to 0.1%) of mesalamine and 0.03 to 1.4 mg/kg/day of N-acetyl-5-aminosalicylic acid.8.4 Pediatric Use
The safety and effectiveness of mesalamine delayed-release tablets have not been established in patients weighing less than 24 kg.
Pediatric use information is approved for Takeda Pharmaceuticals U.S.A., Inc.’s LIALDA (mesalamine) delayed-release tablets. However, due to Takeda Pharmaceuticals U.S.A., Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
Clinical trials of mesalamine did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias (i.e., agranulocytosis, neutropenia, and pancytopenia) in patients who were 65 years or older who were taking mesalamine-containing products such as mesalamine compared to younger patients. Monitor complete blood cell counts and platelet counts in elderly patients during treatment with mesalamine.
Systemic exposures are increased in elderly subjects [see Clinical Pharmacology (12.3)].
In general, consider the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients when prescribing mesalamine. Consider starting at the low end of the dosing range for induction in elderly patients [see Dosage and Administration (2), Use in Specific Populations (8.6)].
8.6 Renal Impairment
Mesalamine is known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Evaluate renal function in all patients prior to initiation and periodically while on mesalamine tablets therapy. Monitor patients with known renal impairment or history of renal disease or taking nephrotoxic drugs for decreased renal function and mesalamine-related adverse reactions. Discontinue mesalamine if renal function deteriorates while on therapy. [see Warnings and Precautions (5.1), Adverse Reactions (6.2), Drug Interactions (7.1)].
-
10 OVERDOSAGE
Mesalamine is an aminosalicylate, and symptoms of salicylate toxicity may include nausea, vomiting, abdominal pain, tachypnea, hyperpnea, tinnitus, and neurologic symptoms (headache, dizziness, confusion, seizures). Severe intoxication with salicylates may lead to electrolyte and blood pH imbalance, and potentially end organ (e.g., renal and liver) damage.
There is no specific known antidote for mesalamine overdose; however, conventional therapy for salicylate toxicity may be beneficial in the event of acute overdosage and may include gastrointestinal tract decontamination to prevent further absorption. Correct fluid and electrolyte imbalance by the administration of appropriate intravenous therapy and maintain adequate renal function.
Mesalamine is a pH-dependent, delayed-release product and this factor should be considered when treating a suspected overdose.
-
11 DESCRIPTION
Each mesalamine delayed-release tablet for oral administration contains 1.2 g 5-aminosalicylic acid (5-ASA; mesalamine), an anti-inflammatory agent. Mesalamine also has the chemical name 5-amino-2-hydroxybenzoic acid and its structural formula is:
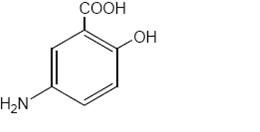
Molecular formula: C 7H 7NO 3
Molecular weight: 153.14Mesalamine, USP is a light tan to pink colored, needle-shaped crystals. Color may darken on exposure to air. It is odorless or may have a slight characteristic odor.
The tablet is coated with a pH dependent polymer film, which breaks down at or above pH 6.8, normally in the terminal ileum where mesalamine then begins to be released from the tablet core. The tablet core contains mesalamine with hydrophilic excipients and provides for extended release of mesalamine.
Each mesalamine delayed-release tablet, USP intended for oral administration contains 1.2 g of mesalamine. In addition, each tablet contains the following inactive ingredients: carboxymethylcellulose sodium, colloidal silicon dioxide, hypromellose, iron oxide red, iron oxide yellow, magnesium stearate, methacrylic acid copolymer, microcrystalline cellulose, polyethylene glycol, sodium starch glycolate, triethyl citrate, talc and titanium dioxide.
The Product meets USP Dissolution Test 4.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of mesalamine is not fully understood, but it appears to have a topical anti-inflammatory effect on the colonic epithelial cells. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase and lipoxygenase pathways, is increased in patients with ulcerative colitis, and it is possible that mesalamine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin production in the colon.
12.3 Pharmacokinetics
Absorption
The total absorption of mesalamine from mesalamine delayed-release tablets 2.4 g or 4.8 g given once daily for 14 days to healthy subjects was found to be approximately 21% to 22 % of the administered dose.Gamma-scintigraphy studies have shown that a single dose of mesalamine delayed-release tablets 1.2 g (one tablet) passed intact through the upper gastrointestinal tract of fasted healthy subjects. Scintigraphic images showed a trail of radio-labeled tracer in the colon, suggesting that mesalamine had distributed through this region of the gastrointestinal tract.
In a single dose study, mesalamine delayed-release tablets 1.2 g, 2.4 g and 4.8 g were administered in the fasted state to healthy subjects. Plasma concentrations of mesalamine were detectable after 2 hours and reached a maximum by 9 to 12 hours on average for the doses studied. The pharmacokinetic parameters are highly variable among subjects (Table 4). Mesalamine systemic exposure in terms of area under the plasma concentration-time curve (AUC) was slightly more than dose proportional between 1.2 g and 4.8 g mesalamine delayed-release tablets. Maximum plasma concentrations (C max) of mesalamine increased approximately dose proportionately between 1.2 g and 2.4 g and sub-proportionately between 2.4 g and 4.8 g of mesalamine delayed-release tablets, with the dose normalized value at 4.8 g representing, on average, 74 % of that at 2.4 g based on geometric means.
Table 4 Mean (SD) Pharmacokinetic Parameters for Mesalamine Following Single Dose Administration of Mesalamine Delayed-Release Tablets Under Fasting Conditions Parameter*of Mesalamine Mesalamine Delayed-Release Tablets 1.2 g
(N=47)Mesalamine Delayed-Release Tablets 2.4 g
(N=48)Mesalamine Delayed-Release Tablets 4.8 g
(N=48)AUC 0-t (ng.h/mL)
9039 †(5054)
20538 (12980)
41434 (26640)
AUC 0-∞ (ng.h/mL)
9578 ‡(5214)
21084 (13185)
44775 §(30302)
C max (ng/mL)
857 (638)
1595 (1484)
2154 (1140)
T max¶(h)
9 #(4 to 32.1)
12 (4 to 34.1)
12 (4 to 34)
T lag¶(h)
2 #(0 to 8)
2 (1 to 4)
2 (1 to 4)
T 1/2(h) (Terminal Phase)
8.56 ‡(6.38)
7.05 Þ(5.54)
7.25 §(8.32)
Food Effects
Administration of a single dose of mesalamine delayed-release tablets 4.8 g with a high fat meal resulted in further delay in absorption, and plasma concentrations of mesalamine were detectable 4 hours following dosing. However, a high fat meal increased systemic exposure of mesalamine (mean C max: increased 91 %; mean AUC: increased 16 %) compared to results in the fasted state. Mesalamine delayed-release tablets were administered with food in the controlled clinical trials [see Dosage and Administration (2)].In a single and multiple dose pharmacokinetic study of mesalamine delayed-release tablets, 2.4 g or 4.8 g was administered once daily with standard meals to 28 healthy subjects per dose group. Plasma concentrations of mesalamine were detectable after 4 hours and were maximal by 8 hours after the single dose. Steady state was achieved generally by 2 days after dosing. Mean AUC at steady state was only modestly greater (1.1- to 1.4-fold) than predictable from single dose pharmacokinetics.
Distribution
Mesalamine is approximately 43% bound to plasma proteins at the concentration of 2.5 mcg/mL.Elimination
Metabolism
The only major metabolite of mesalamine (5-aminosalicylic acid) is N-acetyl-5-aminosalicylic acid. Its formation is brought about by N-acetyltransferase (NAT) activity in the liver and intestinal mucosa cells, principally by NAT-1.Excretion
Excretion of mesalamine is mainly via the renal route following metabolism to N-acetyl-5-aminosalicylic acid (acetylation); however, there is also limited excretion of the parent drug in urine. Of the approximately 21% to 22% of the dose absorbed, less than 8% of the dose was excreted unchanged in the urine after 24 hours, compared with greater than 13% for N-acetyl-5-aminosalicylic acid. The mean renal clearance (CL R) in adults ranged from 1.8 L/h to 2.9 L/h following single dose administration and ranged from 5.5 L/h to 6.4 L/h after a multiple dosing for 14 days. The apparent terminal half-lives for mesalamine and its major metabolite after administration of mesalamine 2.4 g and 4.8 g were, on average, 7 to 9 hours and 8 to 12 hours, respectively.Systemic exposures in adult subjects were inversely correlated with renal function as assessed by estimated creatinine clearance [see Use in Specific Populations (8.6)].
Specific Populations
Geriatric Patients
In a single dose pharmacokinetic study of mesalamine, 4.8 g was administered in the fasted state to 71 healthy male and female subjects (28 young (18 to 35 years); 28 elderly (65 to 75 years); 15 elderly (> 75 years)). Increased age resulted in increased systemic exposure (approximately 2-fold in C max), to mesalamine and its metabolite N-acetyl-5-aminosalicylic acid. Increased age resulted in a slower apparent elimination of mesalamine, though there was high between-subject variability.Table 5 Mean (SD) Pharmacokinetic Parameters for Mesalamine Following Single Dose Administration of Mesalamine Delayed-Release Tablets 4.8 g under Fasting Conditions to Young and Elderly Subjects Arithmetic mean (SD) data are presented, N = Number of subjects; 5-ASA = 5-aminosalicylic acid Parameter of 5-ASA
Young Subjects
(18 to 35 years)
(N=28)
Elderly Subjects
(65 to 75 years)
(N=28)
Elderly Subjects
(>75 years)
(N=15)
AUC 0-t (ng.h/mL)
51570 (23870)
73001 (42608)
65820 (25283)
AUC 0-∞ (ng.h/mL)
58057 *(22429)
89612 †(40596)
63067 ‡(22531)
C max (ng/mL)
2243 (1410)
4999 (4381)
4832 (4383)
t max§(h)
22 (5.98 to 48)
12.5 (4 to 36)
16 (4 to 26)
t lag§(h)
2 (1 to 6)
2 (1 to 4)
2 (2 to 4)
t ½(h), terminal phase
5.68 *(2.83)
9.68 †(7.47)
8.67 ‡(5.84)
Renal clearance (L/h)
2.05 (1.33)
2.04 (1.16)
2.13 (1.20)
Drug Interaction Studies
The potential effect of mesalamine (4.8 g given once daily) on the pharmacokinetics of four commonly used antibiotics were evaluated in healthy subjects. The four antibiotics studied and their dosing regimens were as follows: amoxicillin (single 500 mg dose), ciprofloxacin XR (single 500 mg dose), metronidazole (750 mg twice daily for 3.5 days), and sulfamethoxazole/trimethoprim (800 mg/160 mg twice daily for 3.5 days). The change in C max and AUC of amoxicillin, ciprofloxacin and metronidazole when they were coadministered with mesalamine were all 3% or less. There was an increase of 12% in C max and an increase of 15% in AUC of sulfamethoxazole when sulfamethoxazole/trimethoprim was coadministered with mesalamine. Coadministration of mesalamine did not result in clinically significant changes in the pharmacokinetics of any of the four antibiotics. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 104-week dietary carcinogenicity study in CD-1 mice, mesalamine at doses up to 2500 mg/kg/day was not tumorigenic. This dose is 2.2 times the maximum recommended human dose (based on a body surface area comparison) of mesalamine delayed-release tablets. Furthermore, in a 104-week dietary carcinogenicity study in Wistar rats, mesalamine up to a dose of 800 mg/kg/day was not tumorigenic. This dose is 1.4 times the recommended human dose (based on a body surface area comparison) of mesalamine delayed-release tablets.Mutagenesis
No evidence of mutagenicity was observed in an in vitro Ames test or an in vivo mouse micronucleus test.Impairment of Fertility
No effects on fertility or reproductive performance were observed in male or female rats at oral doses of mesalamine up to 400 mg/kg/day (0.7 times the maximum recommended human dose based on a body surface area comparison).13.2 Animal Toxicology and/or Pharmacology
In animal studies with mesalamine, a 13-week oral toxicity study in mice and 13-week and 52-week oral toxicity studies in rats and cynomolgus monkeys have shown the kidney to be the major target organ of mesalamine toxicity. Oral daily doses of 2400 mg/kg in mice and 1150 mg/kg in rats produced renal lesions including granular and hyaline casts, tubular degeneration, tubular dilation, renal infarct, papillary necrosis, tubular necrosis, and interstitial nephritis. In cynomolgus monkeys, oral daily doses of 250 mg/kg or higher produced nephrosis, papillary edema, and interstitial fibrosis.
-
14 CLINICAL STUDIES
14.1 Adults with Mildly to Moderately Active Ulcerative Colitis
Induction of Remission
Two similarly designed, randomized, double-blind, placebo-controlled trials (Study 1, NCT00503243 and Study 2, NCT00548574) were conducted in 517 adult patients with mildly to moderately active ulcerative colitis. The study population was primarily Caucasian (80%), had a mean age of 42 years (6% age 65 years or older), and was approximately 50% male. Both studies used mesalamine delayed-release tablets dosages of 2.4 g and 4.8 g administered once daily for 8 weeks, except in Study 1 the 2.4 g dosage was administered as two divided doses (i.e., 1.2 g twice daily). The primary efficacy endpoint in both trials was to compare the percentage of patients in remission after 8 weeks of treatment for the mesalamine delayed-release tablets treatment groups versus placebo. Remission was defined as an Ulcerative Colitis Disease Activity Index (UC-DAI) of ≤ 1, with scores of zero for rectal bleeding and for stool frequency, and a sigmoidoscopy score reduction of 1 point or more from baseline.In both studies, the mesalamine delayed-release tablets dosages of 2.4 g and 4.8 g once daily demonstrated superiority over placebo in the primary efficacy endpoint (Table 6). Both mesalamine delayed-release tablets dosages also provided consistent benefit in secondary efficacy parameters, including clinical improvement, clinical remission, and sigmoidoscopic improvement. Both mesalamine delayed-release tablets dosages had similar efficacy profiles.
Table 6 Proportion of Adult Patients with Mildly to Moderately Active Ulcerative Colitis in Remission at Week 8 in Two Double-Blind Placebo-Controlled Induction Trials Dose
Study 1
(n=262)
n/N (%)
Study 2
(n=255)
n/N (%)
Mesalamine Delayed-Release Tablets 2.4 g/day
30/88 (34)
34/84 (41)
Mesalamine Delayed-Release Tablets 4.8 g/day
26/89 (29)
35/85 (41)
Placebo
11/85 (13)
19/86 (22)
Maintenance of Remission
A multicenter, randomized, double-blind, active comparator study (Study 3, NCT00151892) was conducted in a total of 826 adult patients in remission from ulcerative colitis. Patients were randomized in a 1:1 ratio to receive either mesalamine 2.4 g administered once daily or another mesalamine delayed-release product administered as 0.8 g twice daily. The study population had a mean age of 45 years (8% age 65 years or older), were 52% male, and were primarily Caucasian (64%).Maintenance of remission was assessed using a modified UC-DAI. For this trial, maintenance of remission was based on maintaining endoscopic remission defined as a modified UC-DAI endoscopy subscore of ≤1. An endoscopy subscore of 0 represented normal mucosal appearance with intact vascular pattern and no friability or granulation. For this trial the endoscopy score definition of 1 (mild disease) was modified such that it could include erythema, decreased vascular pattern, and minimal granularity; however, it could not include friability.
The proportion of patients who maintained remission at Month 6 in this study using mesalamine delayed-release tablets 2.4 g once daily (84%) was similar to the comparator (82%).
Pediatric use information is approved for Takeda Pharmaceuticals U.S.A., Inc.’s LIALDA (mesalamine) delayed-release tablets. However, due to Takeda Pharmaceuticals U.S.A., Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Mesalamine Delayed-Release Tablets USP, 1.2 g are pale red-brown, oval-shaped, biconvex, bevel film-coated tablets debossed with the '711' on one side and plain on other side and are supplied as follows:
Unit dose packages of 30 (5 x 6) NDC 60687-397-25Storage
Store at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature].FOR YOUR PROTECTION: Do not use if blister is torn or broken.
-
17 PATIENT COUNSELING INFORMATION
Renal Impairment
Inform patients that mesalamine may decrease their renal function, especially if they have known renal impairment or are taking nephrotoxic drugs, and periodic monitoring of renal function will be performed while they are on therapy. Advise patients to complete all blood tests ordered by their healthcare provider [see Warnings and Precautions (5.1)].Mesalamine-Induced Acute Intolerance Syndrome and Other Hypersensitivity Reactions
Instruct patients to stop taking mesalamine and report to their healthcare provider if they experience new or worsening symptoms of acute intolerance syndrome (cramping, abdominal pain, bloody diarrhea, fever, headache, and rash) or other symptoms suggestive of mesalamine-induced hypersensitivity [see Warnings and Precautions (5.2, 5.3)].Hepatic Failure
Advise patients with known liver disease to contact their healthcare provider if they experience signs or symptoms of worsening liver function [see Warnings and Precautions (5.4)].Severe Cutaneous Adverse Reactions
Inform patients of the signs and symptoms of severe cutaneous adverse reactions. Instruct patients to stop taking mesalamine delayed-release tablets and report to their healthcare provider at first appearance of a severe cutaneous adverse reaction or other sign of hypersensitivity [see Warnings and Precautions (5.5)].Upper Gastrointestinal Tract Obstruction
Advise patients to contact their healthcare provider if they experience signs and symptoms of upper gastrointestinal tract obstruction [see Warnings and Precautions (5.6)].Photosensitivity
Advise patients with pre-existing skin conditions to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors [see Warnings and Precautions (5.7)].Nephrolithiasis
Instruct patients to drink an adequate amount of fluids during treatment in order to minimize the risk of kidney stone formation and to contact their healthcare provider if they experience signs or symptoms of a kidney stone (e.g., severe side or back pain, blood in the urine) [see Warnings and Precautions (5.8)].Blood Disorders
Inform elderly patients and those taking azathioprine or 6-mercaptopurine of the risk for blood disorders and the need for periodic monitoring of complete blood cell counts and platelet counts while on therapy. Advise patients to complete all blood tests ordered by their healthcare provider [see Drug Interactions (7.2), Use in Specific Populations (8.5)].Administration
Instruct patients:- Swallow mesalamine delayed-release tablets whole; do not split or crush.
- Take mesalamine delayed-release tablets with food [see Clinical Pharmacology (12.3)].
- Drink an adequate amount of fluids [see Warnings and Precautions (5.8)].
- Intact, partially intact, and/or tablet shells have been reported in the stool and contact their healthcare provider if this occurs repeatedly.
-
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see How Supplied section) contain drug product from Zydus Pharmaceuticals USA Inc. as follows:
(1.2 g / 30 UD) NDC 60687-397-25 packaged from NDC 68382-711Distributed by:
American Health Packaging
Columbus, OH 432178439725/0323F
-
Package/Label Display Panel – Carton – 1.2 g

NDC 60687- 397-25
ONCE DAILY
Mesalamine
Delayed-Release Tablets, USP1.2 g
30 Tablets (5 x 6) Rx Only
Each Delayed-Release Tablet Contains:
Mesalamine, USP...........................................................1.2 gUsual Dosage: See package insert for full prescribing
information.Store at 20° to 25°C (68° to 77°F); excursions permitted
between 15° to 30°C (59° to 86°F) [see USP Controlled
Room Temperature].Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or
broken.The drug product contained in this package is from
NDC # 68382-711, Zydus Pharmaceuticals (USA) Inc.Distributed by:
American Health Packaging
Columbus, Ohio 43217739725
0439725/0321 - Package/Label Display Panel – Blister – 1.2 g
-
INGREDIENTS AND APPEARANCE
MESALAMINE
mesalamine tablet, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60687-397(NDC:68382-711) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MESALAMINE (UNII: 4Q81I59GXC) (MESALAMINE - UNII:4Q81I59GXC) MESALAMINE 1.2 g Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID (UNII: 1CS02G8656) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red (BROWN) Score no score Shape OVAL (OVAL) Size 10mm Flavor Imprint Code 711 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60687-397-25 30 in 1 BOX, UNIT-DOSE 10/11/2018 1 NDC:60687-397-95 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091640 10/11/2018 Labeler - American Health Packaging (929561009) Establishment Name Address ID/FEI Business Operations American Health Packaging 929561009 repack(60687-397)



