Label: ALLERGY RELIEF- loratadine solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 49614-174-26 - Packager: Medicine Shoppe International Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 23, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
- Directions
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
-
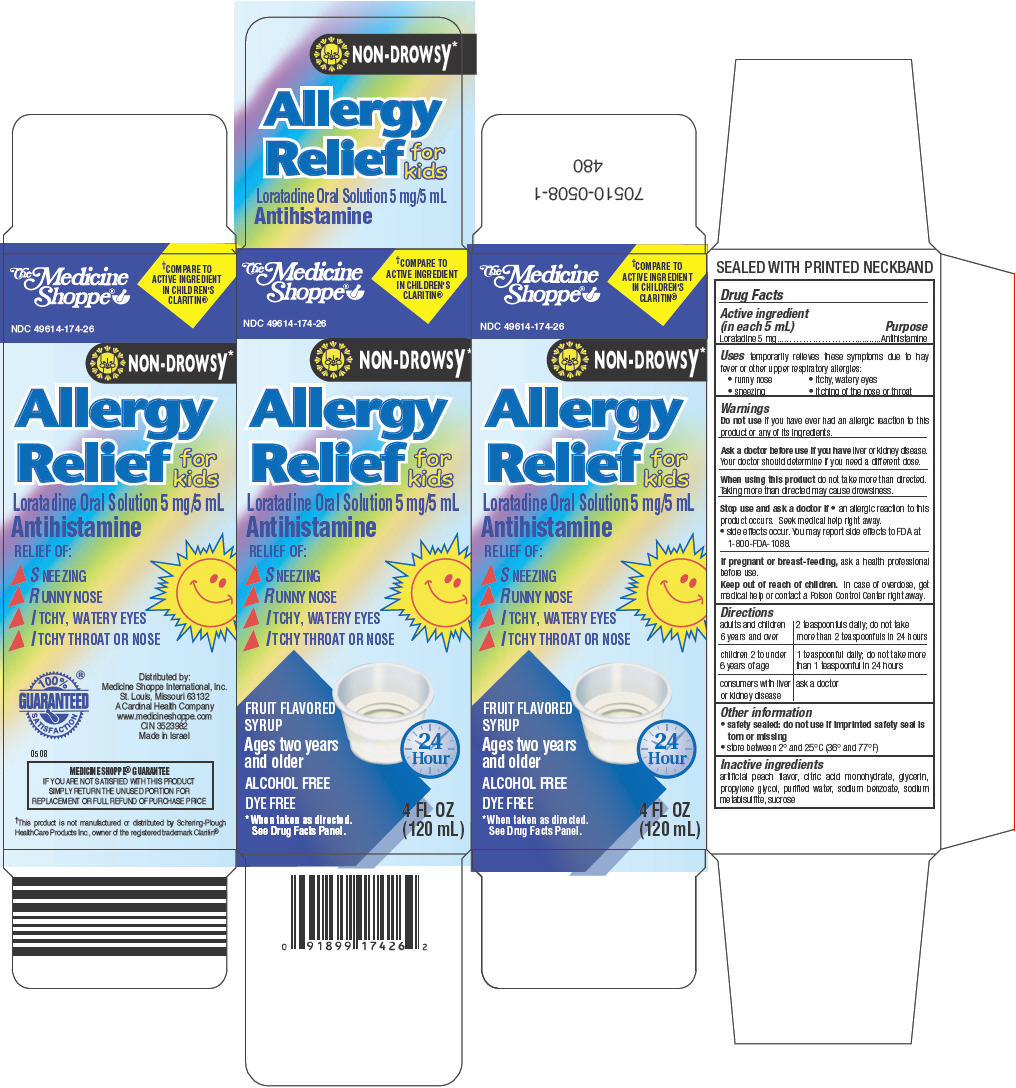
PRINCIPAL DISPLAY PANEL - 120 mL Bottle Carton
The Medicine
Shoppe®†COMPARE TO
ACTIVE INGREDIENT
IN CHILDREN'S
CLARITIN®NDC 49614-174-26
NON-DROWSY*
Allergy
Relief
for
kids
Loratadine Oral Solution 5 mg/5 mL
AntihistamineRELIEF OF:
- ▶
- SNEEZING
- ▶
- RUNNY NOSE
- ▶
- ITCHY, WATERY EYES
- ▶
- ITCHY THROAT OR NOSE
FRUIT FLAVORED
SYRUPAges two years
and olderALCOHOL FREE
DYE FREE
*When taken as directed.
See Drug Facts Panel.24
Hour4 FL OZ
(120 mL)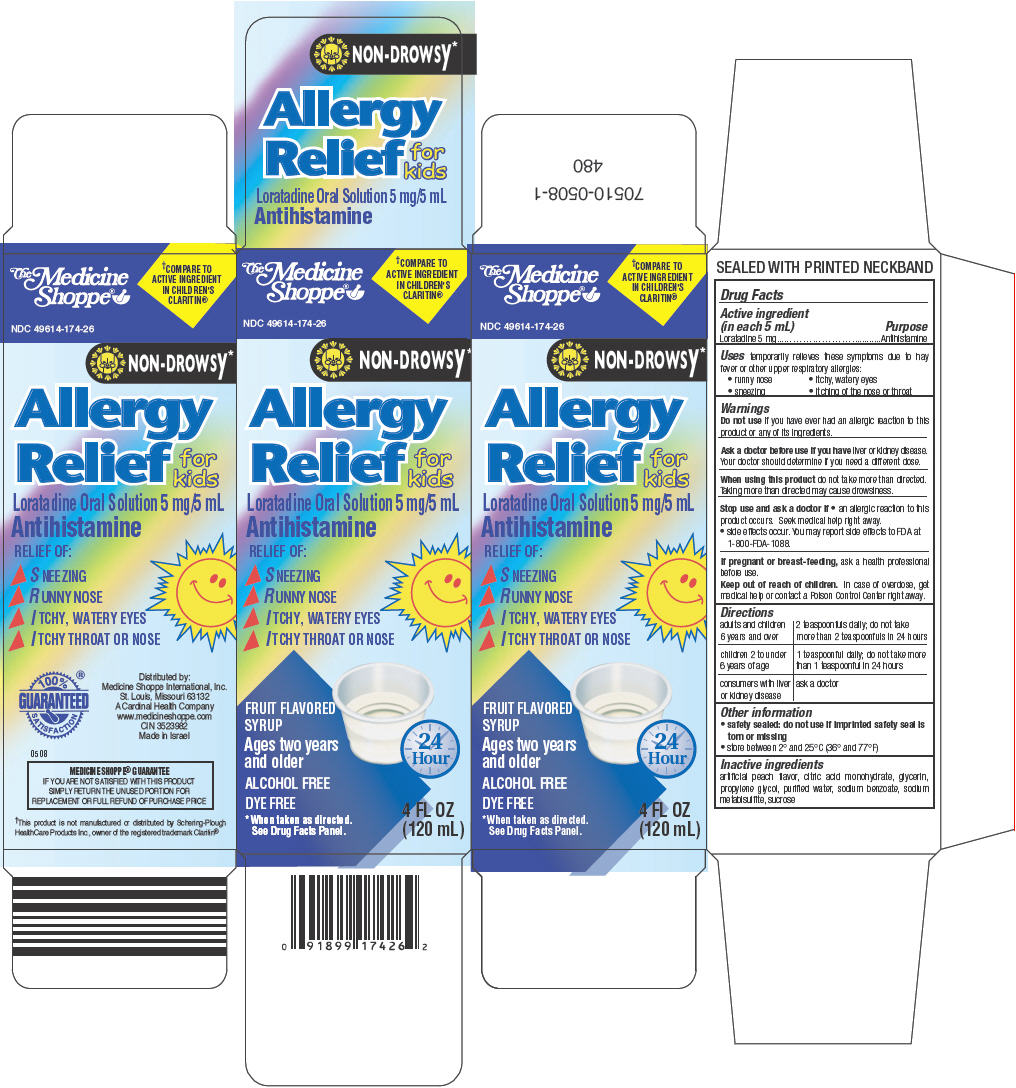
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
loratadine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49614-174 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Loratadine (UNII: 7AJO3BO7QN) (Loratadine - UNII:7AJO3BO7QN) Loratadine 5 mg in 5 mL Inactive Ingredients Ingredient Name Strength citric acid monohydrate (UNII: 2968PHW8QP) glycerin (UNII: PDC6A3C0OX) propylene glycol (UNII: 6DC9Q167V3) water (UNII: 059QF0KO0R) sodium benzoate (UNII: OJ245FE5EU) sodium metabisulfite (UNII: 4VON5FNS3C) sucrose (UNII: C151H8M554) Product Characteristics Color YELLOW (colorless to slightly yellow) Score Shape Size Flavor FRUIT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49614-174-26 1 in 1 CARTON 1 120 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076805 08/20/2004 Labeler - Medicine Shoppe International Inc (071997654) Registrant - Taro Pharmaceuticals U.S.A., Inc. (145186370) Establishment Name Address ID/FEI Business Operations Taro Pharmaceutical Industries Ltd. 600072078 MANUFACTURE(49614-174)