Label: CALCIUM CARBONATE 1250 MG- calcium carbonate tablet
- NDC Code(s): 70369-005-02, 70369-005-03
- Packager: CitraGen Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 31, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
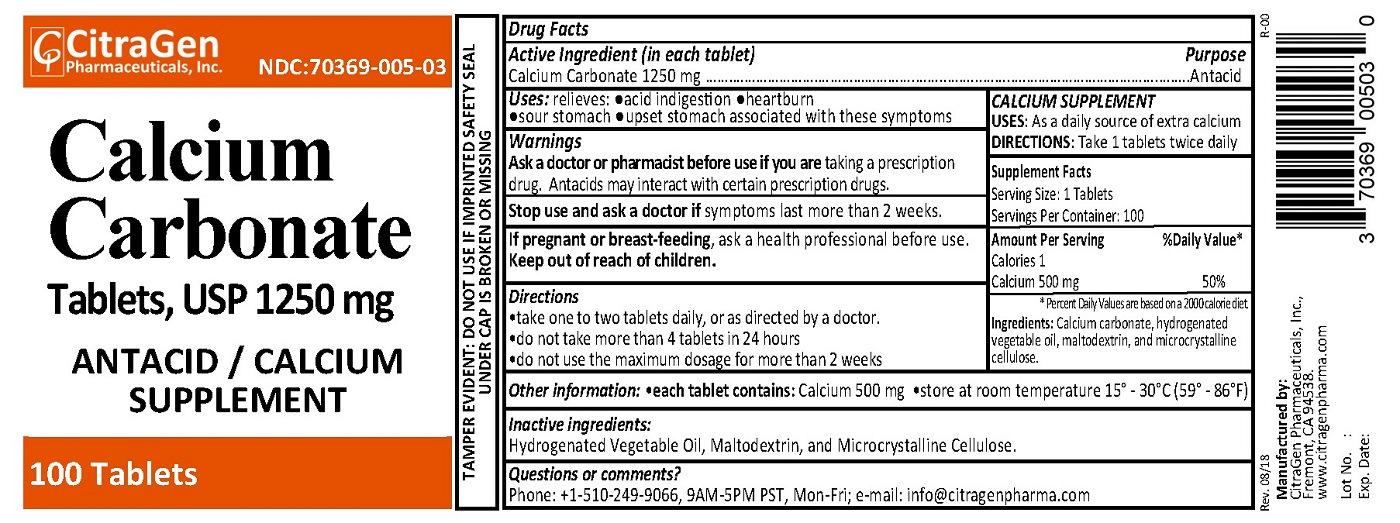
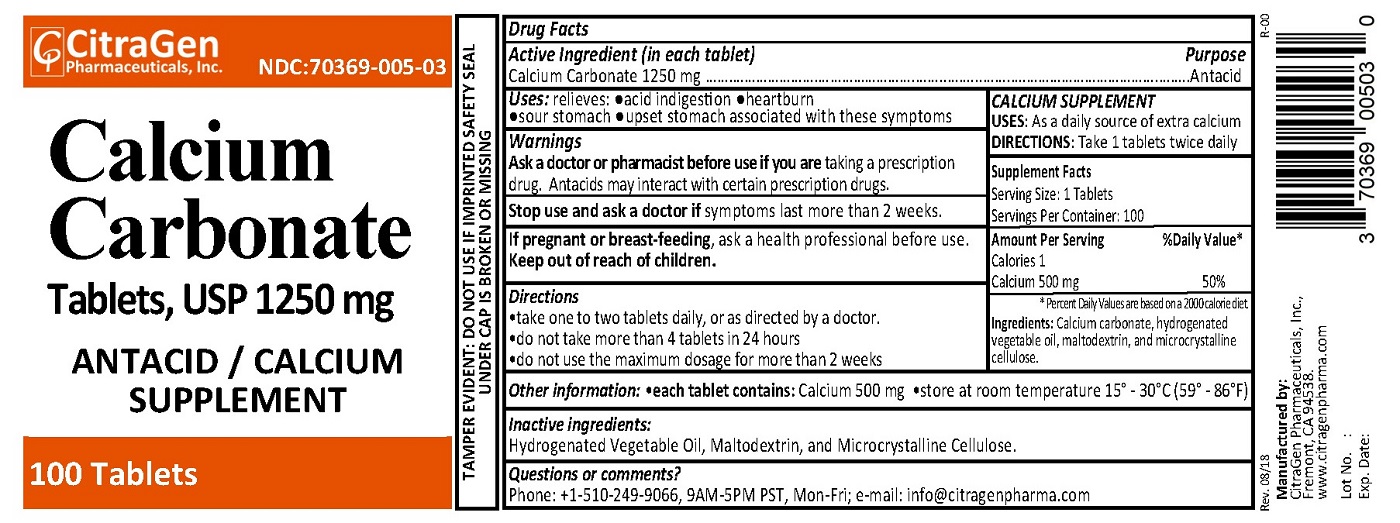
- PRINCIPAL DISPLAY PANEL
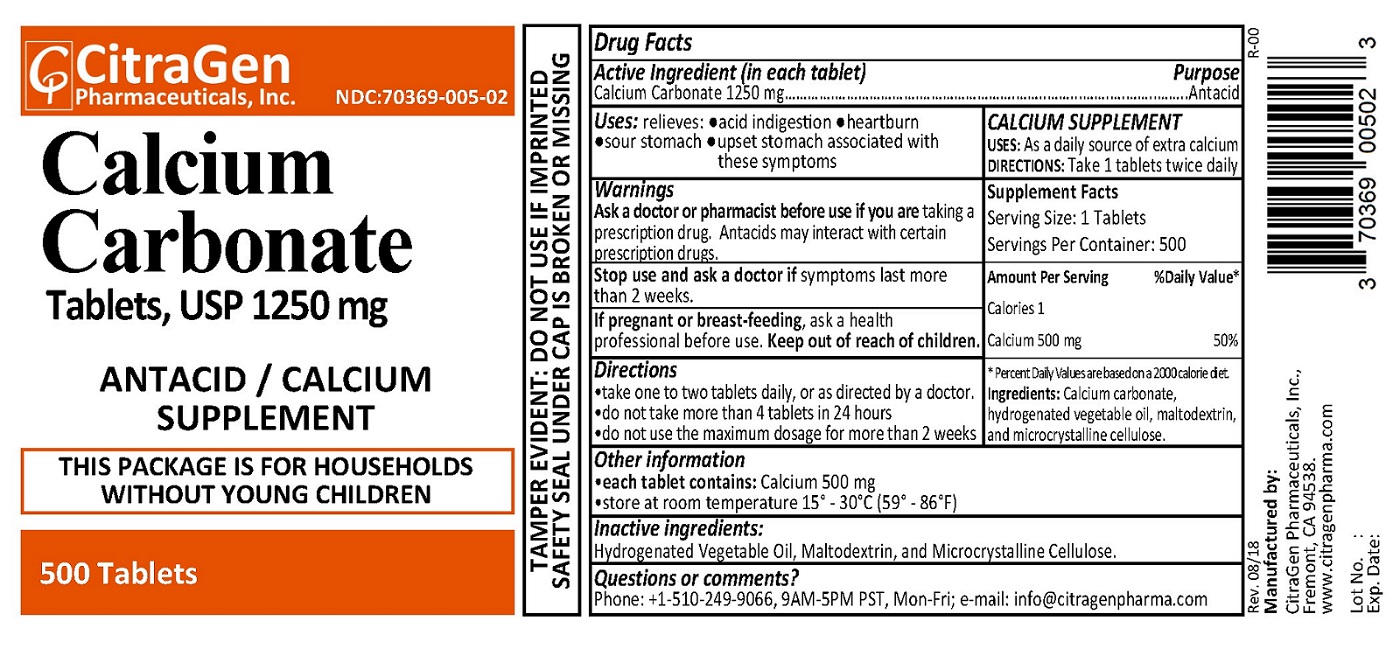
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CALCIUM CARBONATE 1250 MG
calcium carbonate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70369-005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 1250 mg Inactive Ingredients Ingredient Name Strength HYDROGENATED COTTONSEED OIL (UNII: Z82Y2C65EA) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color white Score no score Shape CAPSULE Size 18mm Flavor Imprint Code CG005 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70369-005-02 500 in 1 BOTTLE; Type 0: Not a Combination Product 09/26/2018 2 NDC:70369-005-03 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 09/26/2018 Labeler - CitraGen Pharmaceuticals, Inc. (024949457) Registrant - CitraGen Pharmaceuticals, Inc. (024949457) Establishment Name Address ID/FEI Business Operations CitraGen Pharmaceuticals, Inc. 024949457 manufacture(70369-005)