INFLUENZINUM- influenza a virus a/singapore/infimh-16-0019/2016 ivr-186 (h3n2) hemagglutinin antigen (formaldehyde inactivated), influenza b virus b/maryland/15/2016 bx-69a antigen (formaldehyde inactivated), influenza a virus a/michigan/45/2015 x-275 (h1n1) hemagglutinin antigen (formaldehyde inactivated) pellet
Boiron
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
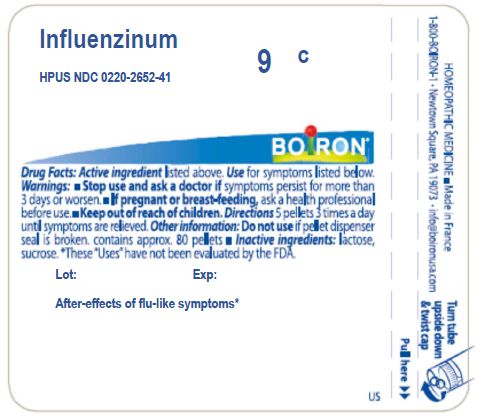
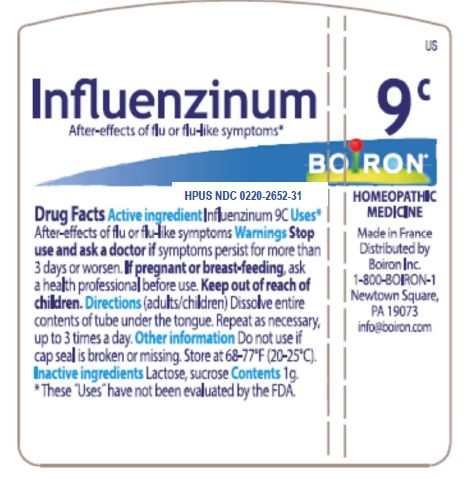
Influenzinum 8X HPUS
A/Michigan/45/2015 (H1N1) pdm09-like virus
A/Singapore/INFIMH-16–0019/2016 (H3N2)-like virus
B/Maryland/15/2016/ BX-69A (B/Colorado/06/2017-like virus (B/Victoria lineage))
Relieves after effects of flu or flu-like symptoms
Relieves after effects of flu or flu-like symptoms
Stop use and ask a doctor if symptoms persist for more than 3 days or worsen
If pregnant or breast-feeding, ask a health professional before use
Unit dose: Dissolve entire contents of the tube under the tongue, repeat as necessary up to 3 times a day
Multi-dose: 5 pellets 3 times a day until symptoms are relieved
Multi-dose:
Do not use if pellet dispenser seal is broken
Approx 80 pellets
These "Uses" have not been evaluated by the FDA
Unit Dose:
Do not use if cap seal is broken or missing
Store at 68-77 degrees F
These "Uses" have not been evaluated by the FDA
Made in France
Distributed by Boiron Inc.
1-800-BOIRON-1
Newtown Square, PA 19073
info@Boiron.com