BUTORPHANOL TARTRATE- butorphanol tartrate injection, solution
Apotex Corp.
----------
BUTORPHANOL TARTATE INJECTION USP
Rx Only
DESCRIPTION
Butorphanol tartrate is a synthetically derived opioid agonist-antagonist analgesic of the phenanthrene series. The chemical name is ( – )-17-(cyclobutylmethyl)morphinan-3,14-diol D- ( – ) - tartrate (1:1) (salt). The molecular formula is C21H29NO2•C4H6O6, which corresponds to a molecular weight of 477.55 and the following structural formula:
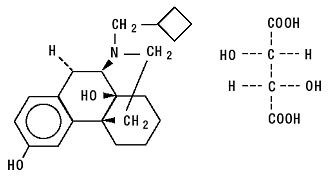
Butorphanol tartrate is a white crystalline substance. The dose is expressed as the tartrate salt. One milligram of the salt is equivalent to 0.68 mg of the free base. The n-octanol/ aqueous buffer partition coefficient of butorphanol is 180:1 at pH 7.5.
Butorphanol tartrate injection is a sterile, parenteral, aqueous solution of butorphanol tartrate for intravenous or intramuscular administration. In addition to 1 or 2 mg of butorphanol tartrate, each mL contains 3.3 mg of citric acid, 7.29 mg sodium citrate dihydrate (equivalent to 6.4 mg sodium citrate, anhydrous), and 6.4 mg of sodium chloride, and 0.1 mg benzethonium chloride (in multiple dose vial only) as a preservative. The pH range is 3.0 to 5.5.
CLINICAL PHARMACOLOGY
General Pharmacology and Mechanism of Action
Butorphanol is a mixed agonist-antagonist with low intrinsic activity at receptors of the µ-opioid type (morphine-like). It is also an agonist at κ-opioid receptors.
Its interactions with these receptors in the central nervous system apparently mediate most of its pharmacologic effects, including analgesia.
In addition to analgesia, CNS effects include depression of spontaneous respiratory activity and cough, stimulation of the emetic center, miosis and sedation. Effects possibly mediated by non-CNS mechanisms include alteration in cardiovascular resistance and capacitance, bronchomotor tone, gastrointestinal secretory and motor activity and bladder sphincter activity.
In an animal model, the dose of the butorphanol tartrate required to antagonize morphine analgesia by 50% was similar to that for nalorphine, less than that for pentazocine and more than that for naloxone.
The pharmacological activity of butorphanol metabolites has not been studied in humans; in animal studies, butorphanol metabolites have demonstrated some analgesic activity.
In human studies of butorphanol (seeClinical Trials), sedation is commonly noted at doses of 0.5 mg or more. Narcosis is produced by 10 to 12 mg doses of butorphanol administered over 10 to 15 minutes intravenously.
Butorphanol, like other mixed agonist-antagonists with a high affinity for the κ-receptor may produce unpleasant psychotomimetic effects in some individuals.
Nausea and/or vomiting may be produced by doses of 1 mg or more administered by any route.
In human studies involving individuals without significant respiratory dysfunction, 2 mg of butorphanol IV and 10 mg of morphine sulfate IV depressed respiration to a comparable degree. At higher doses, the magnitude of respiratory depression with butorphanol is not appreciably increased; however, the duration of respiratory depression is longer. Respiratory depression noted after administration of butorphanol to humans by any route is reversed by treatment with naloxone, a specific opioid antagonist (seeOVERDOSAGE: Treatment section).
Butorphanol tartrate demonstrates antitussive effects in animals at doses less than those required for analgesia.
Hemodynamic changes noted during cardiac catheterization in patients receiving single 0.025 mg/kg intravenous doses of butorphanol have included increases in pulmonary artery pressure, wedge pressure and vascular resistance, increases in left ventricular end diastolic pressure and in systemic arterial pressure.
Pharmacodynamics
The analgesic effect of butorphanol is influenced by the route of administration. Onset of analgesia is within a few minutes for intravenous administration and within 15 minutes for intramuscular injection.
Peak analgesic activity occurs within 30 to 60 minutes following intravenous and intramuscular administration.
The duration of analgesia varies depending on the pain model as well as the route of administration, but is generally 3 to 4 hours with IM and IV doses as defined by the time 50% of patients required remedication. In postoperative studies, the duration of analgesia with IV or IM butorphanol was similar to morphine, meperidine, and pentazocine when administered in the same fashion at equipotent doses (seeClinical Trials).
Pharmacokinetics
Butorphanol tartrate is rapidly absorbed after IM injection and peak plasma levels are reached in 20 to 40 minutes.
Following its initial absorption/distribution phase, the single dose pharmacokinetics of butorphanol by the intravenous and intramuscular routes of administration are similar (see Figure 1).
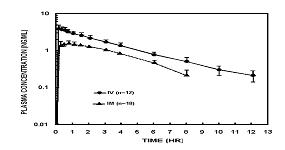
Figure 1—Butorphanol Plasma Levels After IV and IM Administration of 2 mg Dose
Serum protein binding is independent of concentration over the range achieved in clinical practice (up to 7 ng/mL) with a bound fraction of approximately 80%.
The volume of distribution of butorphanol varies from 305 to 901 liters and total body clearance from 52 to 154 liters/hr (see Table 1).
| a) Young subjects (n=24) are from 20 to 40 years old and elderly (n=24) are greater than 65 years of age. | ||||
| b) Area under the plasma concentration-time curve after a 1 mg dose. | ||||
| c) Derived from IV data. | ||||
| Table 1 - | Mean Pharmacokinetic Parameters of Butorphanol in Young and Elderly Subjectsa | |||
|---|---|---|---|---|
| Intravenous | ||||
| Parameters | Young | Elderly | ||
| AUC (inf)b
(hr•ng/mL) | 7.24 (1.57) (4.40-9.77) | 8.71 (2.02) (4.76-13.03) |
||
| Half-life (hr) | 4.56 (1.67) (2.06-8.70) | 5.61 (1.36) (3.25-8.79) |
||
| Volume of Distributionc(L) | 487 (155) (305-901) | 552 (124) (305-737) |
||
| Total Body Clearance (L/hr) | 99 (23) (70-154) | 82 (21) (52-143) |
||
The drug is transported across the blood-brain and placental barriers and into human milk (seePRECAUTIONS: Labor and Delivery and Nursing Mothers sections).
Butorphanol is extensively metabolized in the liver. Metabolism is qualitatively and quantitatively similar following intravenous or intramuscular administration. Oral bioavailability is only 5 to 17% because of extensive first pass metabolism of butorphanol.
The major metabolite of butorphanol is hydroxybutorphanol, while norbutorphanol is produced in small amounts. Both have been detected in plasma following administration of butorphanol, with norbutorphanol present at trace levels at most time points. The elimination half-life of hydroxybutorphanol is about 18 hours and, as a consequence, considerable accumulation (~5-fold) occurs when butorphanol is dosed to steady state (1 mg transnally q6h for 5 days).
Elimination occurs by urine and fecal excretion. When 3H labelled butorphanol is administered to normal subjects, most (70 to 80%) of the dose is recovered in the urine, while approximately 15% is recovered in the feces.
About 5% of the dose is recovered in the urine as butorphanol. Forty-nine percent is eliminated in the urine as hydroxybutorphanol. Less than 5% is excreted in the urine as norbutorphanol.
Butorphanol pharmacokinetics in the elderly differ from younger patients (see Table 1).
In renally impaired patients with creatine clearances <30 mL/min, the elimination half-life was approximately doubled and the total body clearance was approximately one half (10.5 hours [clearance 150 L/h] compared to 5.8 hours [clearance 260 L/h] in healthy subjects). No effect on Cmax or Tmax was observed after a single dose.
After intravenous administration to patients with hepatic impairment, the elimination half-life of butorphanol was approximately tripled and total body clearance was approximately one half (half-life 16.8 hours, clearance 92 L/h) compared to healthy subjects (half-life 4.8 hours, clearance 175 L/h). The exposure of hepatically impaired patients to butorphanol was significantly greater (about 2-fold) than that in healthy subjects.
For further recommendations refer to PRECAUTIONS: Hepatic and Renal Disease, Drug Interactions, and Geriatric Use sections and to the CLINICAL PHARMACOLOGY: Individualization of Dosage section below.
Clinical Trials
The effectiveness of opioid analgesics varies in different pain syndromes. Studies with butorphanol tartrate injection have been performed in postoperative (primarily abdominal and orthopedic) pain and pain during labor and delivery, as preoperative and preanesthetic medication, and as a supplement to balanced anesthesia (see below).
Use in the Management of Pain
Postoperative pain: The analgesic efficacy of butorphanol tartrate injection in postoperative pain was investigated in several double-blind active-controlled studies involving 958 butorphanol-treated patients. The following doses were found to have approximately equivalent analgesic effect: 2 mg butorphanol, 10 mg morphine, 40 mg pentazocine, and 80 mg meperidine.
After intravenous administration of butorphanol tartrate, onset and peak analgesic effect occurred by the time of first observation (30 minutes). After intramuscular administration, pain relief onset occurred at 30 minutes or less, and peak effect occurred between 30 minutes and one hour. The duration of action of butorphanol tartrate injection was 3 to 4 hours when defined as the time necessary for pain intensity to return to pretreatment level or the time to retreatment.
Preanesthetic Medication
Butorphanol tartrate injection (2 mg and 4 mg) and meperidine (80 mg) were studied for use as preanesthetic medication in hospitalized surgical patients. Patients received a single intramuscular dose of either butorphanol or meperidine approximately 90 minutes prior to anesthesia. The anesthesia regimen included barbiturate induction, followed by nitrous oxide and oxygen with halothane or enflurane, with or without a muscle relaxant.
Anesthetic preparation was rated as satisfactory in all 42 patients treated with butorphanol tartrate injection regardless of the type of surgery.
Balanced Anesthesia
Butorphanol tartrate administered intravenously (mean dose 2 mg) was compared to intravenous morphine sulfate (mean dose 10 mg) as premedication shortly before thiopental induction, followed by balanced anesthesia in 50 ASA Class 1 and 2 patients. Anesthesia was then maintained by repeated intravenous doses, averaging 4.6 mg butorphanol tartrate and 22.8 mg morphine per patient.
Anesthetic induction and maintenance were generally rated as satisfactory with both butorphanol tartrate (25 patients) and morphine (25 patients) regardless of the type of surgery performed. Emergence from anesthesia was comparable with both agents.
Labor
(seePRECAUTIONS)
The analgesic efficacy of intravenous butorphanol tartrate was studied in pain during labor. In a total of 145 patients butorphanol tartrate (1 mg and 2 mg) was as effective as 40 mg and 80 mg of meperidine (144 patients) in the relief of pain in labor with no effect on the duration or progress of labor. Both drugs readily crossed the placenta and entered fetal circulation. The condition of the infants in these studies, determined by Apgar scores at 1 and 5 minutes (8 or above) and time to sustained respiration, showed that butorphanol tartrate had the same effects on the infants as meperidine.
In these studies neurobehavioral testing in infants exposed to butorphanol tartrate at a mean of 18.6 hours after delivery, showed no significant differences between treatment groups.
Individualization of Dosage
Use of butorphanol in geriatric patients, patients with renal impairment, patients with hepatic impairment, and during labor requires extra caution (see below and the appropriate sections in PRECAUTIONS).
For pain relief the recommended initial dosage regimen of butorphanol tartrate injection is 1 mg IV or 2 mg IM with repeated doses every 3 to 4 hours, as necessary. This dosage regimen is likely to be effective for the majority of patients. Dosage adjustments of butorphanol tartrate should be based on observations of its beneficial and adverse effects. The initial dose in the elderly and in patients with renal or hepatic impairment should generally be half the recommended adult dose (0.5 mg IV and 1 mg IM). Repeat doses in these patients should be determined by the patient’s response rather than at fixed intervals but will generally be no less than 6 hours (seePRECAUTIONS ).
The usual preoperative dose is 2 mg IM given 60 to 90 minutes before surgery or 2 mg IV shortly before induction. This is approximately equivalent in sedative effect to 10 mg morphine or 80 mg of meperidine. This single preoperative dose should be individualized based on age, body weight, physical status, underlying pathological condition, use of other drugs, type of anesthesia to be used and the surgical procedure involved.
During maintenance in balanced anesthesia the usual incremental dose of butorphanol tartrate is 0.5 mg to 1.0 mg IV. The incremental dose may be higher, up to 0.06 mg/kg (4 mg/70 kg), depending on previous sedative, analgesic, and hypnotic drugs administered. The total dose of butorphanol tartrate will vary; however, patients seldom require less than 4 mg or more than 12.5 mg (approximately 0.06 to 0.18 mg/kg).
As with other opioids of this class, butorphanol tartrate may not provide adequate intraoperative analgesia in every patient or under all conditions. A failure to achieve successful analgesia during balanced anesthesia is commonly reflected by increases in general sympathetic tone. Consequently if blood pressure or heart rate continues to rise, consideration should be given to adding a potent volatile liquid inhalation anesthetic or another intravenous medication.
In labor, the recommended initial dose of butorphanol tartrate is 1 mg or 2 mg IM or IV in mothers with fetuses of 37 weeks gestation or beyond and without signs of fetal distress. Dosage adjustments of butorphanol tartrate in labor should be based on initial response with consideration given to concomitant analgesic or sedative drugs and the expected time of delivery. A dose should not be repeated in less than four hours nor administered less than four hours prior to the anticipated delivery (seePRECAUTIONS ).
INDICATIONS AND USAGE
Butorphanol tartrate injection is indicated for the management of pain when the use of an opioid analgesic is appropriate.
Butorphanol tartrate injection is also indicated as a preoperative or preanesthetic medication, as a supplement to balanced anesthesia, and for the relief of pain during labor.
CONTRAINDICATIONS
Butorphanol tartrate injection is contraindicated in patients hypersensitive to butorphanol tartrate or the preservative benzethonium chloride in the multiple dose vial.
WARNINGS
Patients Dependent on Narcotics
Because of its opioid antagonist properties, butorphanol is not recommended for use in patients dependent on narcotics. Such patients should have an adequate period of withdrawal from opioid drugs prior to beginning butorphanol therapy. In patients taking opioid analgesics chronically, butorphanol has precipitated withdrawal symptoms such as anxiety, agitation, mood changes, hallucinations, dysphoria, weakness and diarrhea.
Because of the difficulty in assessing opioid tolerance in patients who have recently received repeated doses of narcotic analgesic medication, caution should be used in the administration of butorphanol to such patients.
Drug Abuse and Dependence
Drug Abuse: Butorphanol tartrate, by all routes of administration has been associated with episodes of abuse. Of the cases received, there was more report of abuse with the nasal spray formulation than with the injectable formulation.
Physical Dependence, Tolerance, and Withdrawal: Prolonged, continuous use of butorphanol tartrate may result in physical dependence or tolerance (a decrease in response to a given dose). Abrupt cessation of use by patients with physical dependence may result in symptoms of withdrawal.
Note: Proper patient selection, dose and prescribing limitations, appropriate directions for use, and frequent monitoring are important to minimize the risk of abuse and physical dependence (seeDRUG ABUSE AND DEPENDENCE section).
PRECAUTIONS
Head Injury and Increased Intracranial Pressure
As with other opioids, the use of butorphanol in patients with head injury may be associated with carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure, drug-induced miosis, and alterations in mental state that would obscure the interpretation of the clinical course of patients with head injuries. In such patients, butorphanol should be used only if the benefits of use outweigh the potential risks.
Disorders of Respiratory Function or Control
Butorphanol may produce respiratory depression, especially in patients receiving other CNS active agents, or patients suffering from CNS diseases or respiratory impairment.
Hepatic and Renal Disease
In patients with hepatic or renal impairment, the initial dose of butorphanol tartrate injection should generally be half the recommended adult dose (0.5 mg IV and 1 mg IM). Repeat doses in these patients should be determined by the patient’s response rather than at fixed intervals but will generally be no less than 6 hours apart (seeCLINICAL PHARMACOLOGY: Pharmacokinetics and Individualization of Dosage sections).
Cardiovascular Effects
Because butorphanol may increase the work of the heart, especially the pulmonary circuit, the use of butorphanol in patients with acute myocardial infarction, ventricular dysfunction, or coronary insufficiency should be limited to those situations where the benefits clearly outweigh the risk (seeCLINICAL PHARMACOLOGY).
Severe hypertension has been reported rarely during butorphanol therapy. In such cases, butorphanol should be discontinued and the hypertension treated with antihypertensive drugs. In patients who are not opioid dependent, naloxone has also been reported to be effective.
Use in Ambulatory Patients
- Opioid analgesics, including butorphanol, impair the mental and physical abilities required for the performance of potentially dangerous tasks such as driving a car or operating machinery. Effects such as drowsiness or dizziness can appear, usually within the first hour after dosing. These effects may persist for varying periods of time after dosing. Patients who have taken butorphanol should not drive or operate dangerous machinery for at least 1 hour and until the effects of the drug are no longer present.
- Alcohol should not be consumed while using butorphanol. Concurrent use of butorphanol with drugs that affect the central nervous system (e.g., alcohol, barbiturates, tranquilizers, and antihistamines) may result in increased central nervous system depressant effects such as drowsiness, dizziness, and impaired mental function.
- Butorphanol is one of a class of drugs known to be abused and thus should be handled accordingly (seeDRUG ABUSE AND DEPENDENCE section).
Drug Interactions
Concurrent use of butorphanol with central nervous system depressants (e.g., alcohol, barbiturates, tranquilizers, and antihistamines) may result in increased central nervous system depressant effects. When used concurrently with such drugs, the dose of butorphanol should be the smallest effective dose and the frequency of dosing reduced as much as possible when administered concomitantly with drugs that potentiate the action of opioids.
It is not known if the effects of butorphanol are altered by other concomitant medications that affect hepatic metabolism of drugs (erythromycin, theophylline, etc.), but physicians should be alert to the possibility that a smaller initial dose and longer intervals between doses may be needed.
No information is available about the use of butorphanol concurrently with MAO inhibitors.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted in mice and rats given butorphanol tartrate in the diet up to 60 mg/kg/day (180 mg/m2 for mice and 354 mg/m2 for rats). There was no evidence of carcinogenicity in either species in these studies.
Butorphanol was not genotoxic in S. typhimurium or E. coli assays or in unscheduled DNA synthesis and repair assays conducted in cultured human fibroblast cells.
Rats treated orally with 160 mg/kg/day (944 mg/m2) had a reduced pregnancy rate. However, a similar effect was not observed with a 2.5 mg/kg/day (14.75 mg/m2) subcutaneous dose.
Pregnancy
Teratogenic Effects: Pregnancy Category C: Reproduction studies in mice, rats and rabbits during organogenesis did not reveal any teratogenic potential to butorphanol. However, pregnant rats treated subcutaneously with butorphanol at 1 mg/kg (5.9 mg/m2) had a higher frequency of stillbirths than controls. Butorphanol at 30 mg/kg/oral (360 mg/m2) and 60 mg/kg/oral (720 mg/m2) also showed higher incidences of post-implantation loss in rabbits.
There are no adequate and well-controlled studies of butorphanol tartrate in pregnant women before 37 weeks of gestation. Butorphanol tartrate should be used during pregnancy only if the potential benefit justifies the potential risk to the infant.
Labor and Delivery
There have been rare reports of infant respiratory distress/apnea following the administration of butorphanol tartrate injection during labor. The reports of respiratory distress/apnea have been associated with administration of a dose within two hours of delivery, use of multiple doses, use with additional analgesic or sedative drugs, or use in preterm pregnancies (seeOVERDOSAGE: Treatment).
In a study of 119 patients, the administration of 1 mg of IV butorphanol tartrate during labor was associated with transient (10 to 90 minutes) sinusoidal fetal heart rate patterns, but was not associated with adverse neonatal outcomes. In the presence of an abnormal fetal heart rate pattern, butorphanol tartrate should be used with caution.
Nursing Mothers
Butorphanol has been detected in milk following administration of butorphanol tartrate injection to nursing mothers. The amount an infant would receive is probably clinically insignificant (estimated 4 mcg/L of milk in a mother receiving 2 mg IM four times a day).
Pediatric Use
Butorphanol is not recommended for use in patients below 18 years of age because safety and efficacy have not been established in this population.
Geriatric Use
Of the approximately 1500 patients treated with butorphanol tartrate injection in clinical studies, 15% were 61 years of age or older and 1% were 76 years or older.
Due to changes in clearance, the mean half-life of butorphanol is increased by 25% in patients over the age of 65 years. (seeCLINICAL PHARMACOLOGY: Pharmacokinetics section). Elderly patients may be more sensitive to the side effects of butorphanol. There are insufficient efficacy data for patients ≥65 years to determine whether they respond differently from younger patients.
The initial dose of butorphanol tartrate injection recommended for elderly patients should generally be half the recommended adult dose (0.5 mg IV and 1 mg IM). Repeat dose should be determined by the patient’s response rather than at fixed intervals, but will generally be no less than 6 hours apart (seeCLINICAL PHARMACOLOGY: Individualization of Dosage section).
Butorphanol and its metabolites are known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection.
ADVERSE REACTIONS
Clinical Trial Experience
A total of 2446 patients were studied in premarketing clinical trials of butorphanol. Approximately half received butorphanol tartrate injection with the remainder receiving butorphanol tartrate nasal spray. In nearly all cases the type and incidence of side effects with butorphanol by any route were those commonly observed with opioid analgesics.
The adverse experiences described below are based on data from short-term and long-term clinical trials in patients receiving butorphanol by any route. There has been no attempt to correct for placebo effect or to subtract the frequencies reported by placebo-treated patients in controlled trials.
The most frequently reported adverse experiences across all clinical trials with butorphanol tartrate injection and nasal spray were somnolence (43%), dizziness (19%), nausea and/or vomiting (13%). In long-term trials with the nasal spray only, nasal congestion (13%) and insomnia (11%) were frequently reported.
The following adverse experiences were reported at a frequency of 1% or greater in clinical trials and were considered to be probably related to the use of butorphanol:
Body as a Whole: asthenia/lethargy, headache, sensation of heat
Cardiovascular: vasodilation, palpitations
Digestive: anorexia, constipation, dry mouth, nausea and/or vomiting, stomach pain
Nervous: anxiety, confusion, dizziness, euphoria, floating feeling, insomnia, nervousness, paresthesia, somnolence, tremor
Respiratory: bronchitis, cough, dyspnea, epistaxis, nasal congestion, nasal irritation, pharyngitis, rhinitis, sinus congestion, sinusitis, upper respiratory infection
Skin and Appendages: sweating/clammy, pruritus
Special Senses: blurred vision, ear pain, tinnitus, unpleasant taste
The following adverse experiences were reported with a frequency of less than 1% in clinical trials and were considered to be probably related to the use of butorphanol.
Cardiovascular: hypotension, syncope
Nervous: abnormal dreams, agitation, dysphoria, hallucinations, hostility, withdrawal symptoms
Skin and Appendages: rash/hives
Urogenital: impaired urination
The following infrequent additional adverse experiences were reported in a frequency of less than 1% of the patients studied in short-term butorphanol tartrate nasal spray trials and under circumstances where the association between these events and butorphanol administration is unknown. They are being listed as alerting information for the physician.
Body as a Whole: edema
Cardiovascular: chest pain, hypertension, tachycardia
Nervous: depression
Respiratory: shallow breathing
Postmarketing Experience
Postmarketing experience with butorphanol tartrate has shown an adverse event profile similar to that seen during the premarketing evaluation of butorphanol by all routes of administration. Adverse experiences that were associated with the use of butorphanol tartrate nasal spray or butorphanol tartrate injection and that are not listed above have been chosen for inclusion below because of their seriousness, frequency of reporting, or probable relationship to butorphanol. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These adverse experiences include apnea, convulsion, delusion, drug dependence, excessive drug effect associated with transient difficulty speaking and/or executing purposeful movements, overdose, and vertigo. Reports of butorphanol overdose with fatal outcome have usually but not always been associated with ingestion of multiple drugs.
DRUG ABUSE AND DEPENDENCE
Butorphanol tartrate is listed in Schedule IV of the Controlled Substances Act (CSA).
Proper patient selection, dose and prescribing limitations, appropriate directions for use, and frequent monitoring are important to minimize the risk of abuse and physical dependence with butorphanol tartrate. Special care should be exercised in administering butorphanol to patients with a history of drug abuse or to patients receiving the drug on a continuous basis for an extended period.
Clinical Trial Experience
Probable withdrawal symptoms were reported in eight (2.9%) patients receiving butorphanol tartrate nasal spray and no patients receiving placebo in the chronic nonmalignant pain study. Most of these patients abruptly discontinued butorphanol tartrate nasal spray after extended use or high doses. Symptoms suggestive of withdrawal included anxiety, agitation, tremulousness, diarrhea, chills, sweats, insomnia, confusion, incoordination, and hallucinations.
OVERDOSAGE
Clinical Manifestations
The clinical manifestations of butorphanol overdose are those of opioid drugs in general. Consequences of overdose vary with the amount of butorphanol ingested and individual response to the effects of opiates. The most serious symptoms are hypoventilation, cardiovascular insufficiency, coma, and death. Butorphanol overdose may be associated with ingestion of multiple drugs (seeADVERSE REACTIONS: Postmarketing Experience section).
Overdose can occur due to accidental or intentional misuse of butorphanol, especially in young children who may gain access to the drug in the home.
Treatment
The management of suspected butorphanol overdosage includes maintenance of adequate ventilation, peripheral perfusion, normal body temperature, and protection of the airway. Patients should be under continuous observation with adequate serial measures of mental state, responsiveness and vital signs. Oxygen and ventilatory assistance should be available with continual monitoring by pulse oximetry if indicated. In the presence of coma, placement of an artificial airway may be required. An adequate intravenous portal should be maintained to facilitate treatment of hypotension associated with vasodilation.
The use of a specific opioid antagonist such as naloxone should be considered. As the duration of butorphanol action usually exceeds the duration of action of naloxone, repeated dosing with naloxone may be required.
In managing cases of suspected butorphanol overdosage, the possibility of multiple drug ingestion should always be considered.
DOSAGE AND ADMINISTRATION
Factors to be considered in determining the dose are age, body weight, physical status, underlying pathological condition, use of other drugs, type of anesthesia to be used, and surgical procedure involved. Use in elderly, patients with hepatic or renal disease, or in labor requires extra caution (seePRECAUTIONS section andCLINICAL PHARMACOLOGY: Individualization of Dosage section). The following doses are for patients who do not have impaired hepatic or renal function and who are not on CNS active agents.
Use for Pain
Intravenous: The usual recommended single dose for IV administration is 1 mg repeated every 3 to 4 hours as necessary. The effective dosage range, depending on the severity of pain, is 0.5 mg to 2 mg repeated every 3 to 4 hours.
Intramuscular: The usual recommended single dose for IM administration is 2 mg in patients who will be able to remain recumbent, in the event drowsiness or dizziness occurs. This may be repeated every 3 to 4 hours, as necessary. The effective dosage range depending on the severity of pain is 1 mg to 4 mg repeated every 3 to 4 hours. There are insufficient clinical data to recommend single doses above 4 mg.
Use as Preoperative/Preanesthetic Medication
The preoperative medication dosage of butorphanol tartrate injection should be individualized (seeCLINICAL PHARMACOLOGY: Individualization of Dosage section). The usual adult dose is 2 mg IM, administered 60 to 90 minutes before surgery. This is approximately equivalent in sedative effect to 10 mg morphine or 80 mg meperidine.
Use in Balanced Anesthesia
The usual dose of butorphanol tartrate injection is 2 mg IV shortly before induction and/or 0.5 mg to 1 mg IV in increments during anesthesia. The increment may be higher, up to 0.06 mg/kg (4 mg/70 kg), depending on previous sedative, analgesic, and hypnotic drugs administered. The total dose of butorphanol tartrate will vary; however, patients seldom require less than 4 mg or more than 12.5 mg (approximately 0.06 mg/kg to 0.18 mg/kg).
Labor
In patients at full term in early labor a 1 mg to 2 mg dose of butorphanol tartrate IV or IM may be administered and repeated after 4 hours. Alternative analgesia should be used for pain associated with delivery or if delivery is expected to occur within 4 hours.
If concomitant use of butorphanol tartrate with drugs that may potentiate its effects is deemed necessary (seePRECAUTIONS: Drug Interactions section), the lowest effective dose should be employed.
Safety and Handling
Butorphanol tartrate injection is supplied in sealed delivery systems that have a low risk of accidental exposure to health care workers. Ordinary care should be taken to avoid aerosol generation while preparing a syringe for use. Following skin contact, rinsing with cool water is recommended.
The disposal of Schedule IV controlled substances must be consistent with State and Federal Regulations.
HOW SUPPLIED
Butorphanol Tartrate Injection USP, for IM or IV use is available as follows:
2 mg/mL, 2-mL vial, 10 vials per carton
1 mg/mL, 1-mL vial, 10 vials per carton
2 mg/mL, 1-mL vial, 10 vials per carton
2 mg/mL, 10-mL multiple dose vial, individually cartoned.
Storage Conditions
Store at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature].
Protect from light. Discard unused portion. Retain in carton until time of use.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Mfg by:
Apotex Inc.
Toronto, Ontario
Canada M9L 1T9
227409 August 2005
| BUTORPHANOL TARTRATE
butorphanol tartrate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| BUTORPHANOL TARTRATE
butorphanol tartrate injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| BUTORPHANOL TARTRATE
butorphanol tartrate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Apotex Corp. (845263701) |
| Registrant - Apotex Inc. (209429182) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotex Inc. | 255092496 | analysis(60505-0658, 60505-0659, 60505-0660) , manufacture(60505-0658, 60505-0659, 60505-0660) | |