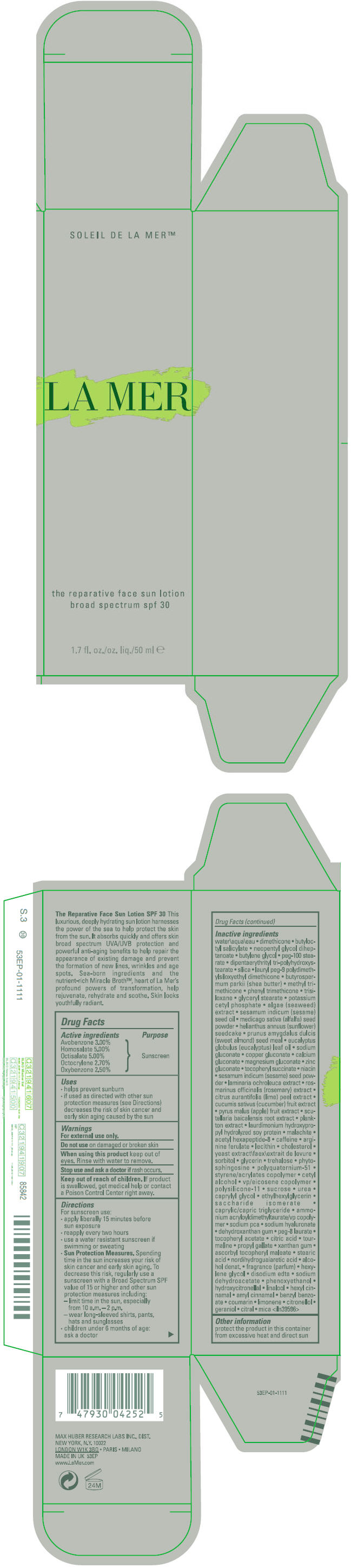
| Inactive Ingredients |
| Ingredient Name | Strength |
| WATER (UNII: 059QF0KO0R) | |
| DIMETHICONE (UNII: 92RU3N3Y1O) | |
| BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) | |
| NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) | |
| BUTYLENE GLYCOL (UNII: 3XUS85K0RA) | |
| PEG-100 STEARATE (UNII: YD01N1999R) | |
| SILICON DIOXIDE (UNII: ETJ7Z6XBU4) | |
| SHEA BUTTER (UNII: K49155WL9Y) | |
| METHYL TRIMETHICONE (UNII: S73ZQI0GXM) | |
| PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) | |
| TRISILOXANE (UNII: 9G1ZW13R0G) | |
| GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) | |
| POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) | |
| SESAME OIL (UNII: QX10HYY4QV) | |
| ALFALFA SEED (UNII: 67PHZ58858) | |
| HELIANTHUS ANNUUS SEEDCAKE (UNII: 482WYF7XLC) | |
| EUCALYPTUS OIL (UNII: 2R04ONI662) | |
| SODIUM GLUCONATE (UNII: R6Q3791S76) | |
| COPPER GLUCONATE (UNII: RV823G6G67) | |
| CALCIUM GLUCONATE (UNII: SQE6VB453K) | |
| MAGNESIUM GLUCONATE (UNII: T42NAD2KHC) | |
| ZINC GLUCONATE (UNII: U6WSN5SQ1Z) | |
| .ALPHA.-TOCOPHEROL SUCCINATE, D- (UNII: LU4B53JYVE) | |
| NIACIN (UNII: 2679MF687A) | |
| SESAME SEED (UNII: 7Y1255HVXR) | |
| LAMINARIA OCHROLEUCA (UNII: 4R2124HE76) | |
| ROSEMARY (UNII: IJ67X351P9) | |
| LIME PEEL (UNII: 544EQK5Q0W) | |
| CUCUMBER (UNII: YY7C30VXJT) | |
| APPLE (UNII: B423VGH5S9) | |
| SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) | |
| ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) | |
| CAFFEINE (UNII: 3G6A5W338E) | |
| CHOLESTEROL (UNII: 97C5T2UQ7J) | |
| YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) | |
| SORBITOL (UNII: 506T60A25R) | |
| GLYCERIN (UNII: PDC6A3C0OX) | |
| TREHALOSE (UNII: B8WCK70T7I) | |
| PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) | |
| CETYL ALCOHOL (UNII: 936JST6JCN) | |
| SUCROSE (UNII: C151H8M554) | |
| UREA (UNII: 8W8T17847W) | |
| CAPRYLYL GLYCOL (UNII: 00YIU5438U) | |
| ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) | |
| SACCHARIDE ISOMERATE (UNII: W8K377W98I) | |
| MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) | |
| AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) | |
| SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) | |
| HYALURONATE SODIUM (UNII: YSE9PPT4TH) | |
| PEG-8 LAURATE (UNII: 762O8IWA10) | |
| .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) | |
| CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) | |
| PROPYL GALLATE (UNII: 8D4SNN7V92) | |
| XANTHAN GUM (UNII: TTV12P4NEE) | |
| ASCORBYL TOCOPHERYL MALEATE (UNII: D2G6259XR5) | |
| STEARIC ACID (UNII: 4ELV7Z65AP) | |
| MASOPROCOL (UNII: 7BO8G1BYQU) | |
| HEXYLENE GLYCOL (UNII: KEH0A3F75J) | |
| EDETATE DISODIUM (UNII: 7FLD91C86K) | |
| SODIUM DEHYDROACETATE (UNII: 8W46YN971G) | |
| PHENOXYETHANOL (UNII: HIE492ZZ3T) | |
| HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) | |
| .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) | |
| .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) | |
| BENZYL BENZOATE (UNII: N863NB338G) | |
| COUMARIN (UNII: A4VZ22K1WT) | |
| .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) | |
| GERANIOL (UNII: L837108USY) | |
| CITRAL (UNII: T7EU0O9VPP) | |
| MICA (UNII: V8A1AW0880) | |