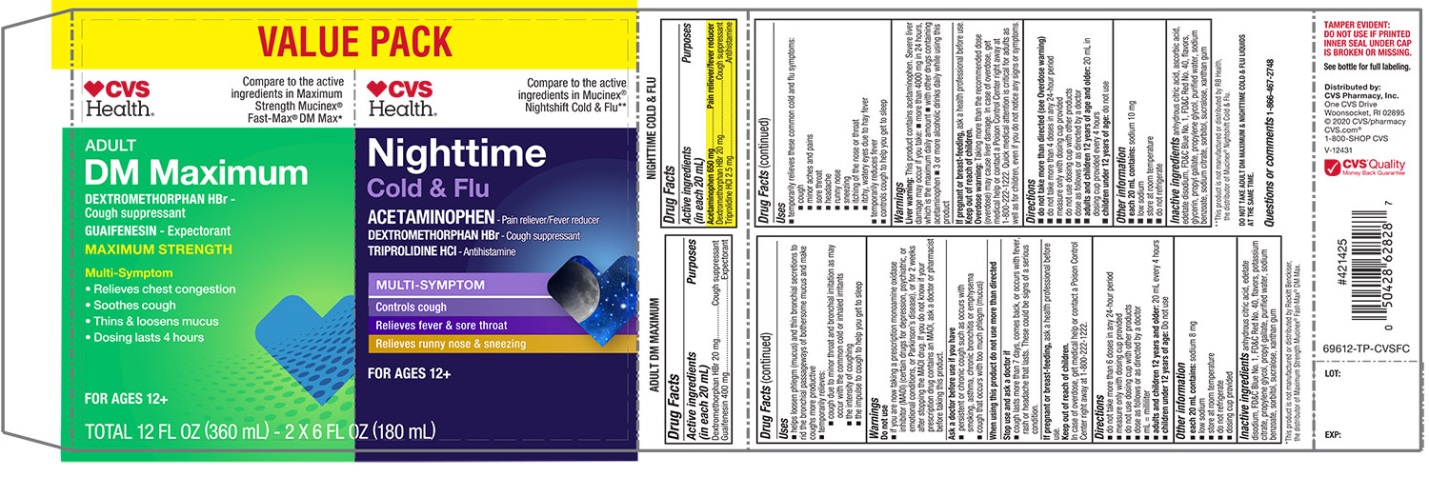
Label: CVS MUCUS RELIEF DM AND OVERNIGHT COLD AND FLU- dextromethorphan hbr, guaifenesin, acetaminophen and triprolidine hcl kit
- NDC Code(s): 69842-696-12
- Packager: CVS
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 12, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
Active ingredients (in each 20 mL)
Maximum Strength Mucus Relief DM MaxPurposes Dextromethorphan HBr 20 mg
Cough suppressant
Guaifenesin 400 mg
Expectorant
Active ingredients (in each 20 mL) Purposes Nighttime Cold & Flu Acetaminophen 650 mg
Pain reliever/fever reducer
Dextromethorphan HBr 20 mg
Cough suppressant
Triprolidine HCl 2.5 mg
Antihistamine
-
Uses
MAXIMUM STRENGTH MUCUS RELIEF DM
- •
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- •
- temporarily relieves
- •
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- •
- the intensity of coughing
- •
- the impulse to cough to help you get to sleep
-
Warnings
Do not use
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- ▪
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- ▪
- cough that occurs with too much phlegm (mucus)
-
Directions
- •
- do not take more than 6 doses in any 24-hour period
- •
- measure only with dosing cup provided
- •
- do not use dosing cup with other products
- •
- dose as follows or as directed by a doctor
- •
- mL = milliliter
- •
- adults and children 12 years and older: 20 mL every 4 hours
- •
- children under 12 years of age: Do not use
- Other information
- Inactive ingredients (Maximum strength mucus relief DM)
- Uses (Nighttime Cold and Flu)
-
Warnings
Liver warnings: This product contains acetaminophen. Severe liver damage may occur if you take\
- ▪
- more than 4000 mg in 24 hours, which is the maximum daily amount
- ▪
- with other drugs containing acetaminophen
- ▪
- 3 or more alcoholic drinks daily while using this product
If pregnant or breast feeding
Ask a health professional before use
Keep Out of Reach of Children
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Centre right away at 1-800-222-1222.
Quick medical attention is critical for adults as well as for children, even if you do not notice any signs
Directions
- ▪
- do not take more than directed (see overdose warnings
- ▪
- do not take more than 4 doses in any 24-hour period
- ▪
- measure only with dosing cup provided
- ▪
- do not use dosing cup with other products
- ▪
- dose as follows or as directed by a doctor
- ▪
- adults and children 12 years of age and older: 20 ml in dosing cup provided every 4 hours
- ▪
- children under 12 years of age: do not use
- Inactive ingredients (Overnight Cold & Flu)
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL - Kit Carton
VALUE PACK
NDC 69842-696-12
Compare to the active ingredients Maximum Strength Mucinex® Fast Max® DM Max*
Mucus Relief DM
Dextromethorphan HBr • Cough Suppressant
Guaifenesin • Expectorant
MAXIMUM STRENGTHMULTI-SYMPTOM
- •
- Controls Cough
- •
- Relieves chest congestion
- •
- Thins & loosens mucus
- •
- 4 hour dosing
For Ages 12+
*This product is not manufactured or distributed by Reckitt Benckiser, the distributor of Maximum Strength Mucinex® Fast-Max® DM Max.
Compare to Mucinex® Nightshift Cold & Flu Active Ingredients**
Overnight Cold & Flu
ACETAMINOPHEN • PAIN RELIEVER/FEVER REDUCER
DEXTROMETHORPHAN HBR • COUGH SUPPRESSANTTRIPROLIDINE HCL • ANTIHISTAMINE
Night Time
Relief for Better Morning
Maximum Strength per 4-hour dose
- •
- Cough
- •
- Fever
- •
- Sore Throat
- •
- Runny Nose
- •
- Sneezing
For Ages 12+
2 – 6 FL OZ (180 mL) BOTTLES / TOTAL 12 FL OZ (360 mL)
††These product is not manufactured or distributed by Reckitt Benckister Health, distributor of Maximum Strength Mucinex® Fast Max© DM Max & Mucinex© Nightshift Cold & Flu.
DO NOT TAKE ADULT DM MAXIMUM & NIGHTTIME COLD & FLU LIQUIDS AT THE SAME TIME.
TAMPER EVIDENT: DO NOT USE IF PRINTED INNER SEAL UNDER CAP IS BROKEN OR MISSING.;
See bottle for full labeling
Distributed By:
CVS Pharmacy, Inc.
One CVS Drive
Woonsocket, RI 02895
© 2020 CVS/pharmacy
CVS.com®
1-800-SHOP CVS
V-12431
CVS® Quality
Money Back Guarantee
-
INGREDIENTS AND APPEARANCE
CVS MUCUS RELIEF DM AND OVERNIGHT COLD AND FLU
dextromethorphan hbr, guaifenesin, acetaminophen and triprolidine hcl kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-696 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-696-12 1 in 1 CARTON; Type 0: Not a Combination Product 03/30/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 180 mL Part 2 1 BOTTLE 180 mL Part 1 of 2 MAXIMUM STRENGTH MUCUS RELIEF DM
dextromethorphan hbr and guaifenesin solutionProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dextromethorphan Hydrobromide (UNII: 9D2RTI9KYH) (Dextromethorphan - UNII:7355X3ROTS) Dextromethorphan Hydrobromide 20 mg in 20 mL Guaifenesin (UNII: 495W7451VQ) (Guaifenesin - UNII:495W7451VQ) Guaifenesin 400 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C Blue No. 1 (UNII: H3R47K3TBD) FD&C Red No. 40 (UNII: WZB9127XOA) POTASSIUM CITRATE (UNII: EE90ONI6FF) propylene glycol (UNII: 6DC9Q167V3) propyl gallate (UNII: 8D4SNN7V92) water (UNII: 059QF0KO0R) sodium benzoate (UNII: OJ245FE5EU) sorbitol (UNII: 506T60A25R) sucralose (UNII: 96K6UQ3ZD4) xanthan gum (UNII: TTV12P4NEE) Product Characteristics Color BLUE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 180 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 03/30/2020 Part 2 of 2 OVERNIGHT COLD AND FLU
acetaminophen, dextromethorphan hbr and triprolidine hcl solutionProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Acetaminophen (UNII: 362O9ITL9D) (Acetaminophen - UNII:362O9ITL9D) Acetaminophen 650 mg in 20 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL TRIPROLIDINE HYDROCHLORIDE (UNII: YAN7R5L890) (TRIPROLIDINE - UNII:2L8T9S52QM) TRIPROLIDINE HYDROCHLORIDE 2.5 mg in 20 mL Inactive Ingredients Ingredient Name Strength anhydrous citric acid (UNII: XF417D3PSL) edetate disodium (UNII: 7FLD91C86K) FD&C Blue No. 1 (UNII: H3R47K3TBD) FD&C Red No. 40 (UNII: WZB9127XOA) glycerin (UNII: PDC6A3C0OX) propyl gallate (UNII: 8D4SNN7V92) propylene glycol (UNII: 6DC9Q167V3) water (UNII: 059QF0KO0R) sodium benzoate (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) sorbitol (UNII: 506T60A25R) sucralose (UNII: 96K6UQ3ZD4) xanthan gum (UNII: TTV12P4NEE) Product Characteristics Color BLUE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 180 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 03/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 03/30/2020 Labeler - CVS (062312574)