CAMILIA- matricaria recutita, rhubarb, phytolacca americana root liquid
Laboratoires Boiron
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
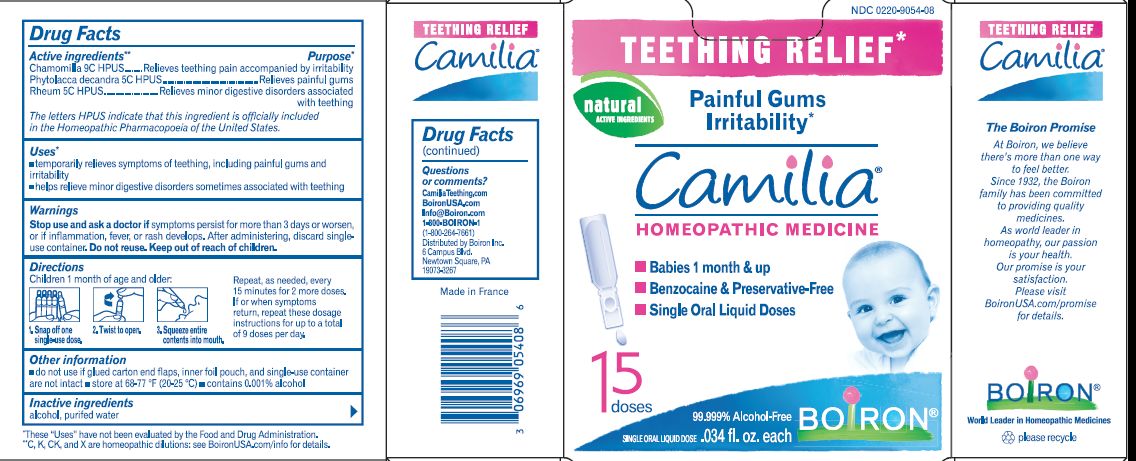
Camilia 15 Dose
Temporarily relieves symptoms of teething, including painful gums and irritability.
Helps relieve minor digestive disorders sometimes associated with teething.
Children 1 month of age and older.
Snap off one single-use dose.
Twist cap to open.
Squeeze entire contents into mouth.
Repeat as needed, every 15 minutes, for 2 more doses.
Chamomilla 9C Relieves teething pain accompanied by irritability
Phytolacca decandra 5C Relieves painful gums
Rheum 5C Relieves minor digestive disorders associated with teething
Questions, Comments?
www.boironusa.com
info@boironusa.com
1-800-BOIRON-1 (1-800-264-7661)
Boiron Information Center
6 Campus Boulevard
Newtown Square, PA 19073-3267
| CAMILIA
matricaria recutita, rhubarb, phytolacca americana root liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Laboratoires Boiron (282560473) |
| Registrant - Boiron Inc. (014892269) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boiron | 383674934 | manufacture(0220-9054) | |