ALLERGY- diphenhydramine hcl tablet, film coated
GREENBRIER INTERNATIONAL, INC.
----------
Assured 44-329-Delisted
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- itchy, watery eyes
- sneezing
- runny nose
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- sneezing
- runny nose
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Directions
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 times in 24 hours
| adults and children 12 years and over | 1 to 2 tablets |
| children 6 to under 12 years | 1 tablet |
| children under 6 years | do not use |
Other information
- each tablet contains: calcium 30 mg
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- protect from moisture
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C red #27 aluminum lake, dicalcium phosphate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, silicon dioxide, stearic acid, talc, titanium dioxide
Principal Display Panel
ASSURED™
COMPARE TO ACTIVE INGREDIENT OF
BENADRYL® ALLERGY ULTRATAB® TABLETS*
Allergy
• Diphenhydramine HCl 25 mg
Antihistamine
Sneezing, Runny Nose, Itchy Throat,
Itchy, Watery Eyes
Actual Size
36 tablets
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed
by Johnson & Johnson Corporation, owner of
the registered trademark Benadryl® Allergy
ULTRATAB® Tablets.
50844 REV1016D32907
Distributed by:
Greenbrier International, Inc.
Chesapeake, VA 23320
Assured 44-329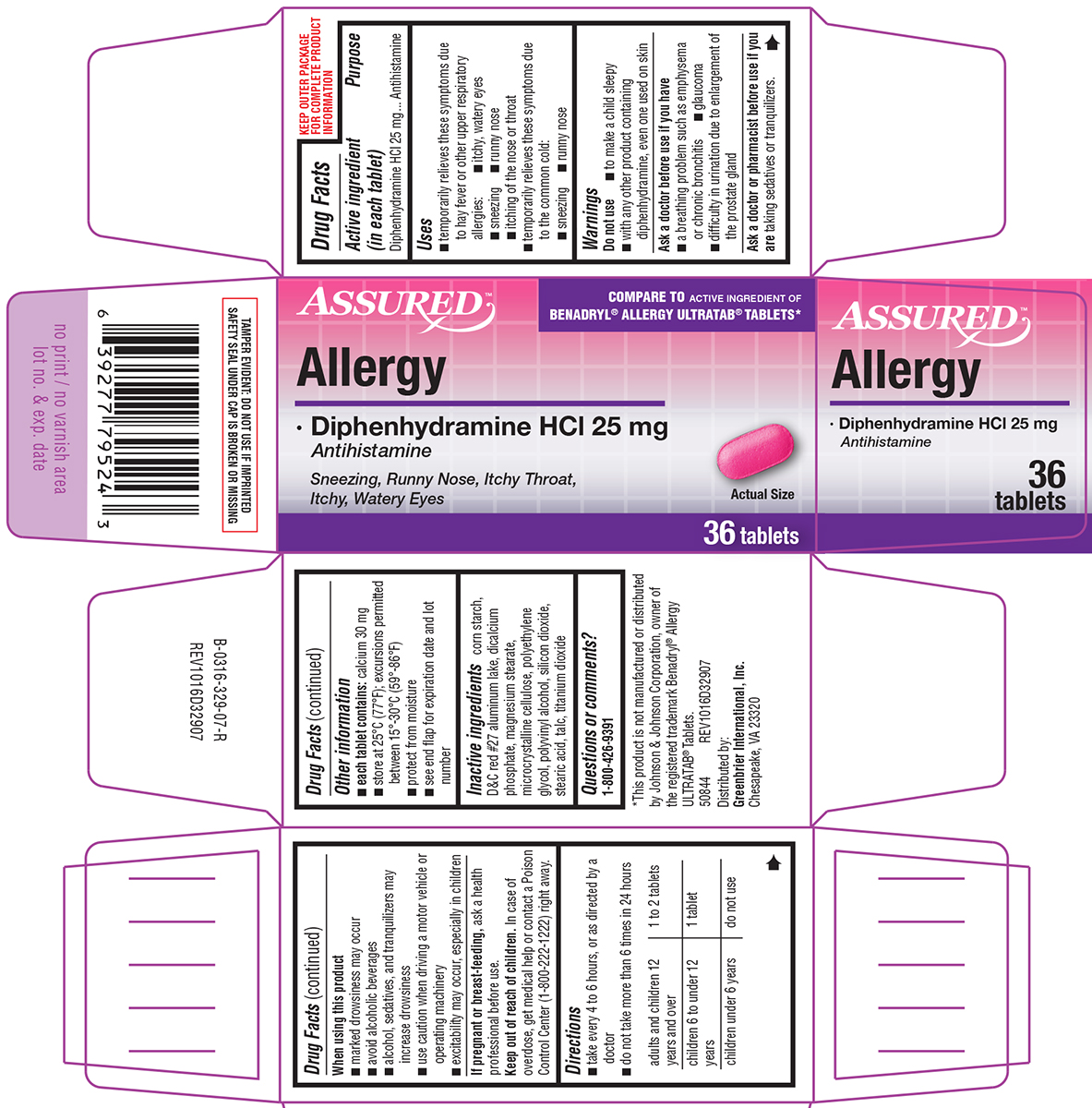
| ALLERGY
diphenhydramine hcl tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - GREENBRIER INTERNATIONAL, INC. (610322518) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(33992-0329) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | manufacture(33992-0329) , pack(33992-0329) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(33992-0329) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | manufacture(33992-0329) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(33992-0329) | |