Label: HIBERIX (haemophilus b conjugate vaccine- tetanus toxoid conjugate kit
-
NDC Code(s):
58160-726-15,
58160-816-01,
58160-816-03,
58160-816-05, view more58160-817-01, 58160-817-02, 58160-817-05, 58160-818-11
- Packager: GlaxoSmithKline Biologicals SA
- Category: VACCINE LABEL
Drug Label Information
Updated December 20, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HIBERIX safely and effectively. See full prescribing information for HIBERIX.
HIBERIX [Haemophilus b Conjugate Vaccine (Tetanus Toxoid Conjugate)] for injection, for intramuscular use
Initial U.S. Approval: 2009RECENT MAJOR CHANGES
Dosage and Administration (2)
12/2023
INDICATIONS AND USAGE
HIBERIX is a vaccine indicated for active immunization for the prevention of invasive disease caused by Haemophilus influenzae type b. HIBERIX is approved for use in children aged 6 weeks through 4 years (prior to fifth birthday). (1)
DOSAGE AND ADMINISTRATION
For intramuscular administration only.
A 4-dose series (either 0.5 mL or approximately 0.5 mL each, depending on the presentation) given by intramuscular injection (2.1):
- •
- Primary series: One dose each at 2, 4, and 6 months of age. The first dose may be given as early as 6 weeks of age.
- •
- Booster: One dose at 15 through 18 months of age.
HIBERIX is supplied in 2 presentations:
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
Severe allergic reaction (e.g., anaphylaxis) after a previous dose of any H. influenzae type b- or tetanus toxoid-containing vaccine or any component of HIBERIX. (4)
WARNINGS AND PRECAUTIONS
- •
- If Guillain-Barré syndrome has occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the decision to give HIBERIX should be based on potential benefits and risks. (5.1)
- •
- Syncope (fainting) can occur in association with administration of injectable vaccines, including HIBERIX. Procedures should be in place to avoid falling injury and to restore cerebral perfusion following syncope. (5.2)
- •
- Apnea following intramuscular vaccination has been observed in some infants born prematurely. Decisions about when to administer an intramuscular vaccine, including HIBERIX, to infants born prematurely should be based on consideration of the individual infant’s medical status, and the potential benefits and possible risks of vaccination. (5.3)
ADVERSE REACTIONS
Common solicited adverse reactions (≥20%) were pain and redness at the injection site, irritability, drowsiness, fever, loss of appetite, fussiness, and restlessness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule
2.2 Presentations and Reconstitution
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome
5.2 Syncope
5.3 Apnea in Premature Infants
5.4 Preventing and Managing Allergic Vaccine Reactions
5.5 Altered Immunocompetence
5.6 Interference with Laboratory Tests
5.7 Tetanus Immunization
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Interference with Laboratory Tests
7.2 Concomitant Vaccine Administration
7.3 Immunosuppressive Therapies
8 USE IN SPECIFIC POPULATIONS
8.4 Pediatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Immunological Evaluation
14.2 Concomitant Vaccine Administration
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 Storage before Reconstitution
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For intramuscular use only.
2.1 Dose and Schedule
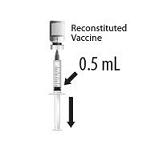
After reconstitution, a single dose of HIBERIX is either 0.5 mL or approximately 0.5 mL depending on the presentation [see Dosage and Administration (2.2)].
HIBERIX is administered as a 4-dose series. The series consists of a primary immunization course of 3 doses administered at 2, 4, and 6 months of age, followed by a booster dose administered at 15 through 18 months of age. The first dose may be given as early as 6 weeks of age.
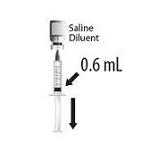
2.2 Presentations and Reconstitution
HIBERIX is supplied in 2 presentations as follows:
Vial and Vial Presentation
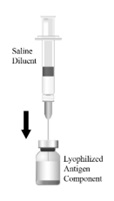
The vial and vial presentation includes a vial of Lyophilized Antigen Component and a vial of Sterile Saline Diluent.
Vial and Prefilled Syringe Presentation
The vial and prefilled syringe presentation includes a vial of Lyophilized Antigen Component and a prefilled syringe of Sterile Saline Diluent.
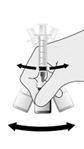
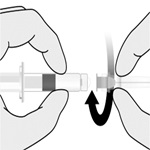
For both presentations reconstitute the Lyophilized Antigen Component only with the accompanying Sterile Saline Diluent to form HIBERIX.
Instructions for Vial and Vial Presentation
Instructions for Vial and Prefilled Syringe Presentation
2.3 Administration
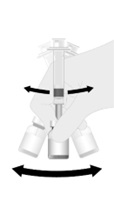
The reconstituted vaccine should be a clear and colorless solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, the vaccine should not be administered.
Administer HIBERIX intramuscularly immediately after reconstitution.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Severe allergic reaction (e.g., anaphylaxis) after a previous dose of any H. influenzae type b- or tetanus toxoid-containing vaccine or any component of the vaccine is a contraindication to administration of HIBERIX [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome
If Guillain-Barré syndrome has occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the decision to give any tetanus toxoid-containing vaccine, including HIBERIX, should be based on careful consideration of the potential benefits and possible risks.
5.2 Syncope
Syncope (fainting) can occur in association with administration of injectable vaccines, including HIBERIX. Syncope can be accompanied by transient neurological signs such as visual disturbance, paresthesia, and tonic-clonic limb movements. Procedures should be in place to avoid falling injury and to restore cerebral perfusion following syncope.
5.3 Apnea in Premature Infants
Apnea following intramuscular vaccination has been observed in some infants born prematurely. Decisions about when to administer an intramuscular vaccine, including HIBERIX, to infants born prematurely should be based on consideration of the individual infant’s medical status, and the potential benefits and possible risks of vaccination.
5.4 Preventing and Managing Allergic Vaccine Reactions
Prior to administration, the healthcare provider should review the patient's immunization history for possible vaccine hypersensitivity. Epinephrine and other appropriate agents used for the control of immediate allergic reactions must be immediately available should an acute anaphylactic reaction occur.
5.5 Altered Immunocompetence
Safety and effectiveness of HIBERIX in immunosuppressed children have not been evaluated. If HIBERIX is administered to immunosuppressed children, including children receiving immunosuppressive therapy, the expected immune response may not be obtained.
5.6 Interference with Laboratory Tests
Urine antigen detection may not have a diagnostic value in suspected disease due to H. influenzae type b within 1 to 2 weeks after receipt of a H. influenzae type b-containing vaccine, including HIBERIX [see Drug Interactions (7.1)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared with rates in the clinical trials of another vaccine and may not reflect the rates observed in practice. There is the possibility that broad use of HIBERIX could reveal adverse reactions not observed in clinical trials.
Across clinical trials, common solicited adverse reactions (≥20%) were pain and redness at the injection site, irritability, drowsiness, fever, loss of appetite, fussiness, and restlessness.
Study 1: In a randomized, controlled clinical trial conducted in the U.S., children were vaccinated with HIBERIX (n = 2,963), a U.S.-licensed monovalent Haemophilus b Conjugate Vaccine (Control PRP-T) (Sanofi Pasteur SA) (n = 520), or a U.S.-licensed combined Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Inactivated Poliovirus and Haemophilus b Conjugate Vaccine (DTaP-IPV/Hib) (Sanofi Pasteur Ltd.) (n = 520) at 2, 4, and 6 months of age. HIBERIX and Control PRP-T (Sanofi Pasteur SA) were administered concomitantly with PEDIARIX (DTaP-HBV-IPV) [Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Hepatitis B (Recombinant) and Inactivated Poliovirus Vaccine] and Pneumococcal 13-valent Conjugate Vaccine (PCV13) (Wyeth Pharmaceuticals Inc.) with Doses 1, 2, and 3 and ROTARIX [Rotavirus Vaccine, Live, Oral] with Doses 1 and 2. DTaP-IPV/Hib was administered concomitantly with PCV13 and ENGERIX-B [Hepatitis B Vaccine (Recombinant)] with Doses 1, 2, and 3 and ROTARIX with Doses 1 and 2. If a birth dose of hepatitis B vaccine was received, ENGERIX-B was given with Doses 1 and 3. In the total population, 51.2% were male; 61% were white, 8% were Asian, 9% were black, and 22% were other racial/ethnic groups.
In Study 1, children received a booster dose of either HIBERIX (n = 2,336), a Haemophilus b Conjugate Vaccine (Control PRP-T) (Sanofi Pasteur SA) (n = 435), or a combined Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Inactivated Poliovirus and Haemophilus b Conjugate Vaccine (DTaP-IPV/Hib) (Sanofi Pasteur Ltd.) (n = 400) at 15 to 18 months of age (mean age: 15.6 months) following primary vaccination at 2, 4, and 6 months of age with the same vaccine. The booster dose of HIBERIX and Control PRP-T (Sanofi Pasteur SA) was administered concomitantly with INFANRIX (DTaP) [Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed].
In 7 additional clinical studies, 1,008 children received HIBERIX as a booster dose following primary vaccination with either HIBERIX (n = 530), Haemophilus b Conjugate Vaccine (Control PRP-T) (Sanofi Pasteur SA) (n = 235), Haemophilus b Conjugate Vaccine (Merck & Co., Inc.) (n = 26), or Haemophilus b Conjugate Vaccine (Wyeth Pharmaceuticals Inc.) (no longer licensed in the U.S., n = 217). None of the studies included a comparator group that received a booster dose with a U.S.-licensed Haemophilus b Conjugate Vaccine. Studies were conducted in Europe, Canada, and Latin America. Across these studies, the mean age of subjects at the time of booster vaccination with HIBERIX ranged from 16 to 19 months. At the time of vaccination, 172 (17.1%) subjects were aged 11 to 14 months, 642 (63.7%) subjects were aged 15 to 18 months, and 194 (19.2%) subjects were aged 19 to 25 months. Approximately half of the subjects were male. Among subjects for whom information on race/ethnicity was available, nearly all subjects were white.
In these 7 studies, HIBERIX was administered concomitantly with non-U.S. formulations (containing 2.5 mg 2-phenoxyethanol per dose as preservative) of one of the following U.S.-licensed vaccines: INFANRIX (DTaP) [Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed], KINRIX (DTaP-IPV) [Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed and Inactivated Poliovirus Vaccine], or PEDIARIX (DTaP-HBV-IPV). In the studies, DTaP-IPV and DTaP-HBV-IPV were administered in dosing regimens not approved in the U.S. Some subjects received DTaP-HBV (GlaxoSmithKline Biologicals, not licensed in U.S.) concomitantly with HIBERIX.
Solicited Adverse Reactions
The reported frequencies of solicited local reactions and general adverse reactions from Study 1 after primary and booster vaccination are presented in Table 1 and Table 2, respectively.
Table 1. Percentage of Children with Solicited Local Reactions and General Adverse Reactions within 4 Days of Primary Series Vaccinationa (at 2, 4, and 6 Months of Age) with HIBERIXb, Control PRP-Tb, or DTaP-IPV/Hibc, Total Vaccinated Cohortd n = All subjects for whom safety data were available.
a Within 4 days of vaccination defined as day of vaccination and the next 3 days.
b Each dose (Doses 1, 2, and 3) of HIBERIX or Control PRP-T (Sanofi Pasteur SA) was concomitantly administered with PEDIARIX (DTaP-HBV-IPV) and PCV13. Doses 1 and 2 were concomitantly administered with ROTARIX.
c Each dose (Doses 1, 2, and 3) of DTaP-IPV/Hib was concomitantly administered with PCV13 and ENGERIX-B with Doses 1, 2, and 3 and ROTARIX with Doses 1 and 2. If a birth dose of hepatitis B vaccine was received, ENGERIX-B was given with Doses 1 and 3.
d Study 1: NCT01000974.
e Local reactions at the injection site for HIBERIX, Control PRP-T, or DTaP-IPV/Hib.
f Grade 3 pain defined as cried when limb was moved/spontaneously painful.
g Grade 3 irritability defined as crying that could not be comforted/prevented normal activity.
h Grade 3 drowsiness defined as prevented normal daily activity.
i Grade 3 loss of appetite defined as did not eat at all.
j Fever defined as ≥100.4°F (≥38.0°C) rectally; Grade 3 fever defined as >103.1°F (>39.5°C) rectally.Adverse Reactions
HIBERIX
%
Control PRP-T
%
DTaP-IPV/Hib
%
Dose
Dose
Dose
1
2
3
1
2
3
1
2
3
Locale
n
2,828
2,668
2,553
498
481
463
492
469
443
Pain
49
45
43
57
53
48
58
50
49
Pain, Grade 3f
4
3
2
9
5
4
9
3
3
Redness
19
25
29
24
32
30
26
31
37
Redness, >20 mm
1
1
1
2
1
0
2
2
2
Swelling
13
15
19
19
22
20
20
24
24
Swelling, >20 mm
2
1
1
4
3
1
4
2
2
General
n
2,830
2,669
2,553
499
480
463
492
469
443
Irritability
69
70
67
76
71
67
73
67
69
Irritability, Grade 3g
4
6
5
8
8
5
6
5
3
Drowsiness
60
54
49
66
56
50
61
52
50
Drowsiness, Grade 3h
2
3
2
4
2
1
4
3
3
Loss of appetite
29
28
28
33
32
27
34
24
24
Loss of appetite, Grade 3i
1
2
2
2
1
0
1
0
1
Fever
14
19
19
16
19
16
12
11
18
Fever, Grade 3j
0
1
1
0
0
1
0
0
1
Table 2. Percentage of Children with Solicited Local Reactions and General Adverse Reactions within 4 Days of Booster Vaccinationa (Dose 4 at 15 through 18 Months of Age) with HIBERIXb, Control PRP-Tb, or DTaP-IPV/Hib, Total Vaccinated Cohortc n = All subjects for whom safety data were available.
Subjects received primary vaccination at 2, 4, and 6 months of age with the same vaccine as the booster dose.
a Within 4 days of vaccination defined as day of vaccination and the next 3 days.
b The booster dose of HIBERIX and Control PRP-T (Sanofi Pasteur SA) was concomitantly administered with INFANRIX (DTaP).
c Study 1: NCT01000974.
d Grade 3 pain defined as cried when limb was moved/spontaneously painful.
Grade 3 redness, swelling defined as >20 mm.
Grade 3 irritability defined as crying that could not be comforted/prevented normal activity.
Grade 3 drowsiness defined as prevented normal daily activity.
Grade 3 loss of appetite defined as did not eat at all.
Grade 3 fever defined as >102.2°F (>39.0°C) axillary.
e Local reactions at the injection site for HIBERIX, Control PRP-T, or DTaP-IPV/Hib.
f Fever defined as ≥99.5°F (≥37.5°C) axillary.Adverse Reactions
HIBERIX
%
Control PRP-T
%
DTaP-IPV/Hib%
Any
Grade 3d
Any
Grade 3d
Any
Grade 3d
Locale
n = 2,224
n = 416
n = 379
Pain
41
1
43
1
43
2
Redness
30
0
31
1
30
3
Swelling
18
1
20
1
20
3
General
n = 2,225
n = 416
n = 379
Irritability
58
2
60
5
53
2
Drowsiness
39
1
39
3
31
0
Loss of appetite
28
1
34
2
22
1
Feverf
15
1
14
1
18
1
In an open-label, multicenter study conducted in Germany (Study 2), 371 children received a booster dose of HIBERIX administered concomitantly with DTaP-HBV-IPV. The mean age at the time of vaccination was 16 months. Subjects in this study had previously received a primary series with either HIBERIX (n = 92), Control PRP-T (Sanofi Pasteur SA) (n = 96), or Haemophilus b Conjugate Vaccine (Wyeth Pharmaceuticals Inc.) (no longer licensed in the U.S.) (n = 183). All subjects previously received 3 doses of DTaP-HBV-IPV. The reported frequencies of solicited local reactions and general adverse reactions are presented in Table 3.
Table 3. Percentage of Children with Solicited Local Reactions and General Adverse Reactions within 4 Days of Booster Vaccinationa (Dose 4) with HIBERIXb Coadministered with DTaP-HBV-IPVc, Intent-to-Treat Cohort (n = 371) n = All subjects for whom safety data were available.
a Within 4 days of vaccination defined as day of vaccination and the next 3 days.
b In this study, 92 subjects previously received 3 doses of HIBERIX, 96 subjects previously received 3 doses of a Control PRP-T (Sanofi Pasteur SA), and 183 subjects previously received 3 doses of a Haemophilus b Conjugate Vaccine that is no longer licensed in the U.S.
c In this study, DTaP-HBV-IPV was given to subjects who previously received 3 doses of DTaP-HBV-IPV. In the U.S., PEDIARIX is approved for use as a 3-dose primary series; use as a fourth consecutive dose is not approved in the U.S.
d Local reactions at the injection site for HIBERIX.
e Grade 3 redness or swelling defined as >20 mm.
f Grade 3 pain defined as causing crying when limb moved.
g Fever defined as ≥100.4°F (≥38.0°C) rectally or ≥99.5°F (≥37.5°C) axillary, oral, or tympanic; Grade 3 fever defined as >103.1°F (>39.5°C) rectally or >102.2°F (>39.0°C) axillary, oral, or tympanic.
h Grade 3 fussiness defined as persistent crying and could not be comforted.
i Grade 3 for these symptoms defined as preventing normal daily activity.%
%
Adverse Reactions
Any
Grade 3
Locald
Redness
25
2e
Pain
21
1f
Swelling
15
2e
General
Feverg
35
4
Fussiness
26
1h
Loss of appetite
23
1i
Restlessness
22
1i
Sleepiness
20
1i
Diarrhea
15
1i
Vomiting
5
1i
Serious Adverse Reactions
In Study 1, one of 2,963 subjects who received HIBERIX and coadministered vaccines given at 2, 4, and 6 months of age experienced a serious adverse reaction which was in temporal association with vaccination and had no alternative plausible causes (convulsion on Day 14 after Dose 1). One of 2,336 subjects who received a booster dose of HIBERIX concomitantly with INFANRIX experienced a serious adverse reaction which was in temporal association with vaccination and had no alternative plausible causes (new onset febrile seizure on Day 1 after Dose 4).
In the 7 additional studies, 2 of 1,008 subjects reported a serious adverse reaction that occurred in the 31-day period following booster immunization with HIBERIX. One subject developed bilateral pneumonia 9 days post-vaccination and one subject experienced asthenia following accidental drug ingestion 18 days post-vaccination.
6.2 Postmarketing Experience
In addition to reports in clinical trials for HIBERIX, the following adverse reactions have been identified during postapproval use of HIBERIX. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to vaccination.
General Disorders and Administration Site Conditions
Extensive swelling of the vaccinated limb, injection site induration.
Immune System Disorders
Allergic reactions (including anaphylactic and anaphylactoid reactions), angioedema.
Nervous System Disorders
Convulsions (with or without fever), hypotonic-hyporesponsive episode (i.e., sudden onset of hypotonia, hyporesponsiveness, and pallor or cyanosis), somnolence, syncope, or vasovagal responses to injection.
Respiratory, Thoracic, and Mediastinal Disorders
Apnea [see Warnings and Precautions (5.3)].
Skin and Subcutaneous Tissue Disorders
Rash, urticaria.
-
7 DRUG INTERACTIONS
7.1 Interference with Laboratory Tests
Haemophilus b capsular polysaccharide derived from Haemophilus b Conjugate Vaccines has been detected in the urine of some vaccinees.1 Urine antigen detection may not have a diagnostic value in suspected disease due to H. influenzae type b within 1 to 2 weeks after receipt of a H. influenzae type b-containing vaccine, including HIBERIX [see Warnings and Precautions (5.6)].
7.2 Concomitant Vaccine Administration
In clinical studies, HIBERIX was administered concomitantly with routinely recommended pediatric vaccines [see Clinical Studies (14.2)].
- 8 USE IN SPECIFIC POPULATIONS
-
11 DESCRIPTION
HIBERIX [Haemophilus b Conjugate Vaccine (Tetanus Toxoid Conjugate)] is a solution for intramuscular injection, supplied as a sterile, lyophilized powder which is reconstituted at the time of use with the accompanying saline diluent. HIBERIX contains Haemophilus b capsular polysaccharide (polyribosyl-ribitol-phosphate [PRP]), a high molecular weight polymer prepared from the H. influenzae type b strain 20,752 grown in a synthetic medium that undergoes heat inactivation and purification. The tetanus toxin, prepared from Clostridium tetani grown in a semi-synthetic medium, is detoxified with formaldehyde and purified. The capsular polysaccharide is covalently bound to the tetanus toxoid. After purification, the conjugate is lyophilized in the presence of lactose as a stabilizer. The diluent for HIBERIX is a sterile saline solution (0.9% sodium chloride) supplied in vials or prefilled syringes.
After reconstitution, each approximately 0.5-mL dose of HIBERIX contains 10 mcg of purified capsular polysaccharide conjugated to approximately 25 mcg of tetanus toxoid, 12.6 mg of lactose, and ≤0.5 mcg of residual formaldehyde.
HIBERIX does not contain a preservative.
The stopper of the vial containing Lyophilized Antigen Component or Sterile Saline Diluent and the tip cap and rubber plunger stopper of the prefilled syringe containing Sterile Saline Diluent are not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
H. influenzae is a gram-negative coccobacillus. Most strains of H. influenzae that cause invasive disease are type b. H. influenzae type b can cause invasive disease such as sepsis and meningitis.
Specific levels of antibodies to polyribosyl-ribitol-phosphate (anti-PRP) have been shown to correlate with protection against invasive disease due to H. influenzae type b. Based on data from passive antibody studies2 and a clinical efficacy study with unconjugated Haemophilus b polysaccharide vaccine3, an anti-PRP concentration of 0.15 mcg/mL has been accepted as a minimal protective level. Data from an efficacy study with unconjugated Haemophilus b polysaccharide vaccine indicate that an anti-PRP concentration of ≥1.0 mcg/mL predicts protection through at least a 1-year period.4,5 These antibody levels have been used to evaluate the effectiveness of Haemophilus b Conjugate Vaccines, including HIBERIX.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Immunological Evaluation
Primary Series Vaccination (Doses 1, 2, and 3)
The immunogenicity of HIBERIX was evaluated in a randomized, controlled trial (Study 1). HIBERIX or control vaccines were administered concomitantly with U.S.-licensed vaccines [see Adverse Reactions (6.1)].
Anti-PRP geometric mean concentrations (GMCs) and seroprotection rates 1 month following Dose 3 of HIBERIX, Control PRP-T (Sanofi Pasteur SA), or DTaP-IPV/Hib are presented in Table 4.
Table 4. Anti-PRP GMCs and Seroprotection Rates 1 Month following 3 Doses of HIBERIX, Control PRP-Ta, or DTaP-IPV/Hibb Administered at 2, 4, and 6 Months of Age, ATP Cohort for Immunogenicityc a U.S.-licensed monovalent Haemophilus b Conjugate Vaccine (Control PRP-T) (Sanofi Pasteur SA).
b U.S.-licensed DTaP-IPV/Hib Vaccine (Sanofi Pasteur Ltd.).
c Study 1: NCT01000974.
d HIBERIX was non-inferior to Control PRP-T for percent of subjects achieving anti-PRP ≥0.15 mcg/mL (lower limit of 95% CI on difference of HIBERIX minus Control PRP-T ≥ predefined limit of -5%).
e The non-inferiority criterion was not met (lower limit of 95% CI for the difference in the percentages of subjects with anti-PRP ≥1.0 mcg/mL between two groups [HIBERIX minus Control PRP-T] was -12.28%, which was lower than the predefined limit of -10%).
f Analyses of anti-PRP immune responses following DTaP-IPV/Hib vaccination were exploratory.Vaccine
n
Anti-PRP GMC
(mcg/mL)
(95% CI)
% Anti-PRP
≥0.15 mcg/mL
(95% CI)
% Anti-PRP
≥1.0 mcg/mL
(95% CI)
HIBERIX
1,590
5.19
(4.77, 5.66)
96.6
(95.6, 97.4)
81.2
(79.2, 83.1)
Control PRP-T
274
6.74
(5.59, 8.13)
96.7d
(93.9, 98.5)
89.8e
(85.6, 93.1)
DTaP-IPV/Hib
253
3.64
(2.89, 4.58)
92.5f
(88.5, 95.4)
78.3f
(72.7, 83.2)
Booster Vaccination (Dose 4)
The immunogenicity of HIBERIX administered as a booster dose at 15 to 18 months of age was evaluated in a subset of children from Study 1 (n = 336) in comparison with U.S.-licensed vaccines following primary vaccination at 2, 4, and 6 months of age [see Adverse Reactions (6.1)]. The booster dose of HIBERIX and Control PRP-T (Sanofi Pasteur SA) was administered concomitantly with INFANRIX.
Antibodies to PRP were measured in sera obtained immediately prior to and 1 month after booster vaccination with HIBERIX or the control vaccines. Anti-PRP GMCs and seroprotection rates are presented in Table 5.
Table 5. Anti-PRP GMCs and Seroprotection Rates prior to and 1 Month following a Booster Dose (Dose 4 at 15 through 18 Months of Age) of HIBERIX, Control PRP-Ta, or DTaP-IPV/Hibb, ATP Cohort for Immunogenicityc a U.S.-licensed monovalent Haemophilus b Conjugate Vaccine (Control PRP-T) (Sanofi Pasteur SA).
b U.S.-licensed DTaP-IPV/Hib Vaccine (Sanofi Pasteur Ltd.).
c Study 1: NCT01000974.
d HIBERIX was non-inferior to Control PRP-T for percent of subjects achieving anti-PRP ≥1.0 mcg/mL (lower limit of 97.5% CI on difference of HIBERIX minus Control PRP-T ≥predefined limit of -10%) at 1 month following the booster dose.
e Analyses of anti-PRP immune responses following DTaP-IPV/Hib vaccination were exploratory.Vaccine
n
Anti-PRP GMC
% Anti-PRP
% Anti-PRP
(mcg/mL)
(95% CI)
≥0.15 mcg/mL
(95% CI)
≥1.0 mcg/mL
(95% CI)
Pre-
Post-
Pre-
Post-
Pre-
Post-
HIBERIX
329-336
0.50
(0.42, 0.59)
48.78
(42.0, 56.66)
75.1
(70.0, 79.7)
100.0
(98.9, 100.0)
32.2
(27.2, 37.6)
99.1
(97.4, 99.8)
Control PRP-T
226-236
0.47
(0.38, 0.57)
40.29
(33.39, 48.63)
76.1
(70.0, 81.5)
99.6
(97.7, 100.0)
27.0
(21.3, 33.3)
97.9d
(95.1, 99.3)
DTaP-IPV/Hib
175-186
0.38
(0.30, 0.48)
37.54
(30.53, 46.16)
66.3
(58.8, 73.2)
100.0
(98.0, 100.0)
25.1
(18.9, 32.2)
98.9e
(96.2, 99.9)
In 6 additional clinical studies, the immune response to HIBERIX administered as a booster dose was evaluated in a total of 415 children aged 12 to 23 months. At the time of vaccination, 30 children were aged 12 to 14 months, 316 children were aged 15 to 18 months, and 69 children were aged 19 to 23 months. Among subjects, 43% to 60% were male. Among subjects for whom information on race/ethnicity was available, nearly all subjects were white. None of the studies included a comparator group that received a booster dose with a U.S.-licensed Haemophilus b Conjugate Vaccine. Characteristics of 3 of these studies are presented in Table 6.
Table 6. Characteristics of 3 Open-Label Booster Immunization Studies of HIBERIX a Administered at a separate site.
b Non-U.S. formulation equivalent to PEDIARIX with the exception of containing 2.5 mg 2-phenoxyethanol per dose as preservative. In the U.S., PEDIARIX is approved for use as a 3-dose primary series; use as a fourth consecutive dose is not approved in the U.S.
c U.S.-licensed Haemophilus b Conjugate Vaccine (Control PRP-T) (Sanofi Pasteur SA).
d Non-U.S. formulation equivalent to KINRIX with the exception of containing 2.5 mg 2-phenoxyethanol per dose as preservative. In the U.S., KINRIX is approved for use as the fifth dose of DTaP and the fourth dose of IPV in children aged 4 to 6 years previously primed with approved dosing regimens of INFANRIX and/or PEDIARIX. The DTaP-IPV dosing regimen is not approved in the U.S.
e Manufactured by GlaxoSmithKline Biologicals (not licensed in the U.S.).Study
Country
Per-Protocol Immunogenicity Cohort
n
Priming History
Booster Vaccination with HIBERIX
Age at Vaccination (months)
Concomitantly Administered Vaccinea
3
Canada
42
DTaP-HBV-IPVb + Haemophilus b Conjugate Vaccinec
at 2, 4, and 6 months of age
16-18
DTaP-HBV-IPVb
4
Canada
64
DTaP-IPVd + HIBERIX
at 2, 4, and 6 months of age
16-19
DTaP-IPVd
5
Germany
108
DTaP-HBVe + HIBERIX
at 3, 4, and 5 months of age
16-23
DTaP-HBVe
Antibodies to PRP were measured in sera obtained immediately prior to and 1 month after booster vaccination with HIBERIX. Geometric mean concentrations and anti-PRP seroprotection rates are presented in Table 7.
Table 7. Anti-PRP GMCs and Seroprotection Rates prior to and 1 Month following a Booster Dose of HIBERIX, Per-Protocol Immunogenicity Cohort GMC = Geometric mean antibody concentration.
n = Number of children for whom serological results were available for the pre- and post-dose immunological evaluations.
Studies 3, 4, and 5 correspond to Studies 3, 4, and 5, respectively in Table 6.
a Canadian study in children aged 16 to 18 months who previously received 3 doses of DTaP-HBV-IPV and Haemophilus b Conjugate Vaccine (Control PRP-T) (Sanofi Pasteur SA). The booster dose of HIBERIX was coadministered with DTaP-HBV-IPV (a fourth consecutive dose of PEDIARIX is not approved in the U.S.). In this study, pre-vaccination sera may have been obtained up to 1 week prior to booster vaccination with HIBERIX.
b Canadian study in children aged 16 to 19 months who previously received 3 doses of DTaP-IPV and HIBERIX. The booster dose of HIBERIX was coadministered with DTaP-IPV. The DTaP-IPV dosing regimen is not approved in the U.S.
c German study in children aged 16 to 23 months who previously received 3 doses of DTaP-HBV (GlaxoSmithKline Biologicals, not licensed in the U.S.) and HIBERIX. The booster dose of HIBERIX was coadministered with DTaP-HBV.Anti-PRP GMC
% Anti-PRP
% Anti-PRP
(mcg/mL)
≥0.15 mcg/mL
≥1.0 mcg/mL
Study
n
Pre-
Post-
Pre-
Post-
Pre-
Post-
3a
42
0.46
59.07
76.2
100
35.7
97.6
4b
63-64
0.25
47.78
71.4
100
12.7
100
5c
108
0.59
96.12
77.8
100
32.4
100
14.2 Concomitant Vaccine Administration
Primary Series Vaccination (Doses 1, 2, and 3)
In U.S. Study 1, subjects who received HIBERIX concomitantly with PEDIARIX (DTaP-HBV-IPV) and PCV13 at 2, 4, and 6 months of age had no evidence for reduced antibody responses relative to the response in control subjects administered Control PRP-T (Sanofi Pasteur SA) concomitantly with PEDIARIX (DTaP-HBV-IPV) and PCV13, to pertussis antigens (GMC to pertussis toxin, filamentous hemagglutinin, and pertactin), diphtheria toxoid (antibody levels ≥0.1 IU/mL), tetanus toxoid (antibody levels ≥0.1 IU/mL), poliovirus types 1, 2, and 3 (antibody levels ≥1:8 to each virus), PCV13 (antibody levels ≥0.2 mcg/mL and GMC to each serotype), or hepatitis B (anti-hepatitis B surface antigen ≥10 mIU/mL). The immune responses to PEDIARIX (DTaP-HBV-IPV) and PCV13 were evaluated 1 month following Dose 3. Subjects in both groups received ROTARIX at 2 and 4 months of age.
Booster Vaccination (Dose 4)
In U.S. Study 1, subjects who received a booster dose of HIBERIX concomitantly with INFANRIX at 15 to 18 months of age had no evidence for reduced antibody responses to pertussis antigens (GMC to pertussis toxin, filamentous hemagglutinin, and pertactin), diphtheria toxoid (antibody levels ≥0.1 IU/mL), and tetanus toxoid (antibody levels ≥0.1 IU/mL), relative to the responses in control subjects administered Control PRP-T (Sanofi Pasteur SA) concomitantly with INFANRIX.
In 7 additional studies, a booster dose of HIBERIX was administered concomitantly with non-U.S. formulations of INFANRIX, KINRIX, and PEDIARIX. Non-U.S. formulations of KINRIX and PEDIARIX were administered in dosing regimens not approved in the U.S.
Sufficient data are not available to confirm lack of interference in immune responses to vaccines other than INFANRIX administered concomitantly with a booster dose of HIBERIX.
-
15 REFERENCES
- 1.
- Rothstein EP, Madore DV, Girone JAC, et al. Comparison of antigenuria after immunization with three Haemophilus influenzae type b conjugate vaccines. Pediatr Infect Dis J. 1991;10:311-314.
- 2.
- Robbins JB, Parke JC, Schneerson R, et al. Quantitative measurement of “natural” and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res. 1973;7:103-110.
- 3.
- Peltola H, Käythy H, Sivonen A, et al. Haemophilus influenzae type b capsular polysaccharide vaccine in children: A double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977;60:730-737.
- 4.
- Käythy H, Peltola H, Karanko V, et al. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983;147:1100.
- 5.
- Anderson P. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1984;149:1034.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
HIBERIX Vial and Vial presentation is supplied in a carton packaged without syringes or needles (NDC 58160-818-11) containing:
- •
- 10 single-dose vials of Lyophilized Antigen Component (NDC 58160-816-01) in a carton: NDC 58160-816-05.
- •
- 10 single-dose vials of Sterile Saline Diluent (NDC 58160-817-01) in a carton: NDC 58160-817-05.
HIBERIX Vial and Prefilled Syringe Presentation is supplied in a carton (NDC 58160-726-15) containing:
- •
- 10 single-dose vials of Lyophilized Antigen Component: NDC 58160-816-03.
- •
- 10 single-dose, prefilled, ungraduated TIP-LOK syringes (Luer Lock syringes) of Sterile Saline Diluent packaged without needles NDC 58160-817-02.
TIP-LOK syringes are to be used with Luer Lock compatible needles.
The stopper of the vial containing Lyophilized Antigen Component or Sterile Saline Diluent and the tip cap and rubber plunger stopper of the prefilled syringe containing Sterile Saline Diluent are not made with natural rubber latex.
16.1 Storage before Reconstitution
Lyophilized Antigen Component vials: Store refrigerated between 2° and 8°C (36° and 46°F). Protect vials from light.
Sterile Saline Diluent prefilled syringes and vials: Store refrigerated or at controlled room temperature between 2° and 25°C (36° and 77°F). Do not freeze. Discard if the diluent has been frozen.
-
17 PATIENT COUNSELING INFORMATION
- •
- Inform parents or guardians of the potential benefits and risks of immunization with HIBERIX.
- •
- Inform parents or guardians about the potential for adverse reactions that have been temporally associated with administration of HIBERIX or other vaccines containing similar components.
- •
- Give parents or guardians the Vaccine Information Statements, which are required by the National Childhood Vaccine Injury Act of 1986 to be given prior to immunization. These materials are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 58160-818-11
HIBERIX
Haemophilus b Conjugate Vaccine (Tetanus Toxoid Conjugate)
Hib
Rx only
For Use from 6 weeks through 4 years of age
Contents (see back panel for storage instructions):
10 Vials of Lyophilized Vaccine
10 Vials of Sterile Saline Diluent (for reconstitution of Lyophilized Vaccine).
Lyophilized Vaccine Made in Singapore
©2020 GSK group of companies or its licensor.
Rev. 11/20
504484
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 58160-726-15
HIBERIX
Haemophilus b Conjugate Vaccine (Tetanus Toxoid Conjugate)
Hib
Rx only
GSK
For Use from 6 weeks through 4 years of age
Contents 10 Doses of HIBERIX:
- •
- 10 Single-dose vials each containing one dose of Lyophilized Antigen Component
- •
- 10 Single-dose prefilled ungraduated TIP-LOK syringes of Sterile Saline Diluent (0.9% NaCl)
After reconstitution, a single dose of HIBERIX is approximately 0.5 mL
TIP-LOK syringes to be used with Luer Lock compatible needles
NEEDLES NOT INCLUDED
©2023 GSK group of companies or its licensor.
Made in Singapore
Rev. 11/23
517080
-
INGREDIENTS AND APPEARANCE
HIBERIX
haemophilus b conjugate vaccine (tetanus toxoid conjugate) kitProduct Information Product Type VACCINE Item Code (Source) NDC:58160-818 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58160-818-11 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 VIAL 5 mL Part 2 10 VIAL 8.5 mL Part 1 of 2 HIBERIX
haemophilus b conjugate vaccine (tetanus toxoid conjugate) injection, powder, for solutionProduct Information Item Code (Source) NDC:58160-816 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HAEMOPHILUS INFLUENZAE TYPE B STRAIN 20752 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN (UNII: C9R35M8XV6) (HAEMOPHILUS INFLUENZAE TYPE B STRAIN 20752 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN - UNII:C9R35M8XV6) HAEMOPHILUS INFLUENZAE TYPE B STRAIN 20752 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN 10 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58160-816-05 10 in 1 CARTON 1 NDC:58160-816-01 0.5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125347 12/21/2015 Part 2 of 2 DILUENT
sterile water solutionProduct Information Item Code (Source) NDC:58160-817 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58160-817-05 10 in 1 PACKAGE 1 NDC:58160-817-01 0.85 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125347 12/21/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125347 12/21/2015 HIBERIX
haemophilus b conjugate vaccine (tetanus toxoid conjugate) kitProduct Information Product Type VACCINE Item Code (Source) NDC:58160-726 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58160-726-15 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 VIAL 5 mL Part 2 10 SYRINGE 8.5 mL Part 1 of 2 HIBERIX
haemophilus b conjugate vaccine (tetanus toxoid conjugate) injection, powder, for solutionProduct Information Item Code (Source) NDC:58160-816 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HAEMOPHILUS INFLUENZAE TYPE B STRAIN 20752 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN (UNII: C9R35M8XV6) (HAEMOPHILUS INFLUENZAE TYPE B STRAIN 20752 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN - UNII:C9R35M8XV6) HAEMOPHILUS INFLUENZAE TYPE B STRAIN 20752 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN 10 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58160-816-03 0.5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125347 12/20/2023 Part 2 of 2 DILUENT
sterile water solutionProduct Information Item Code (Source) NDC:58160-817 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58160-817-02 0.85 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125347 12/20/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125347 12/20/2023 Labeler - GlaxoSmithKline Biologicals SA (372748392)