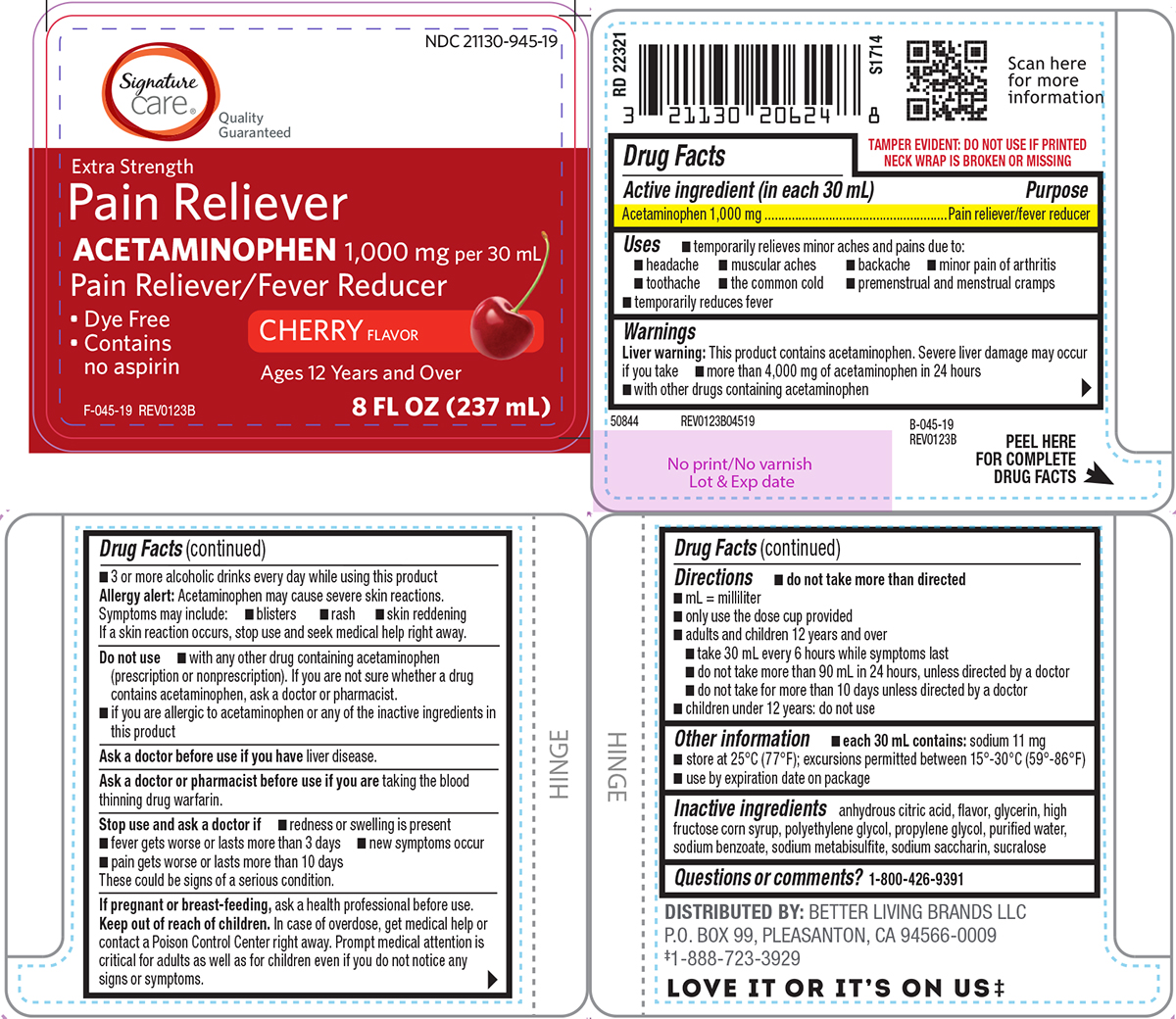
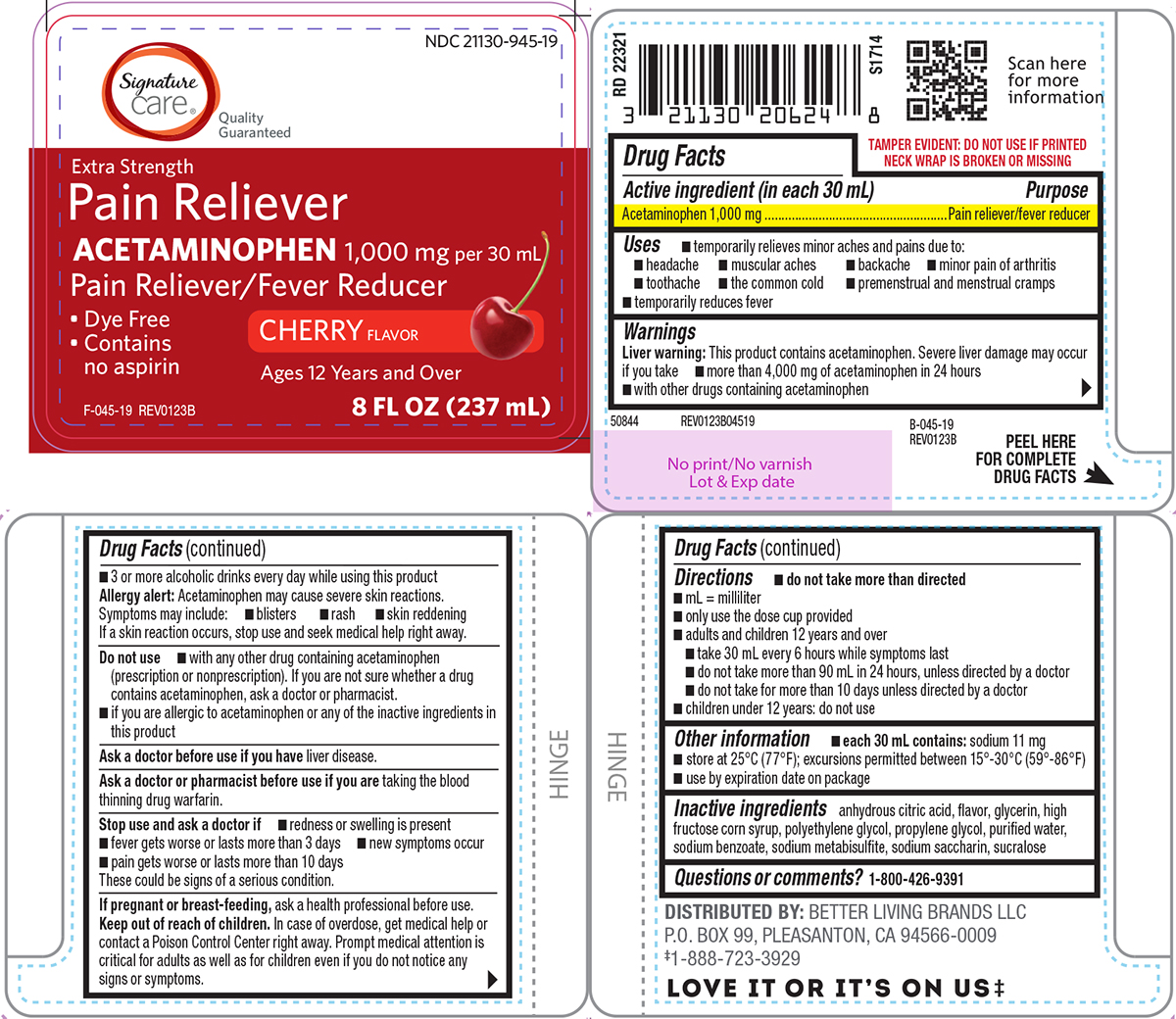
Label: PAIN RELIEVER- acetaminophen solution
- NDC Code(s): 21130-945-19
- Packager: Better Living Brands, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated September 20, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 30 mL)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- blisters
- rash
- skin reddening
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
-
Directions
- do not take more than directed
- mL = milliliter
- only use the dose cup provided
- adults and children 12 years and over
- take 30 mL every 6 hours while symptoms last
- do not take more than 90 mL in 24 hours, unless directed by a doctor
- do not take for more than 10 days unless directed by a doctor
- children under 12 years: do not use
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
Signature
care®
Quality
GuaranteedNDC 21130-945-19
Extra Strength
Pain Reliever
ACETAMINOPHEN 1,000 mg per 30 mLPain Reliever/Fever Reducer
CHERRY FLAVOR
• Dye Free
• Contains
no aspirinF-045-19 REV0123B
8 FL OZ (237 mL)
TAMPER EVIDENT: DO NOT USE IF PRINTED
NECK WRAP IS BROKEN OR MISSINGB-045-19
REV0123B50844 REV0123B04519
DISTRIBUTED BY: BETTER LIVING BRANDS LLC
P.O. BOX 99, PLEASANTON, CA 94566-0009
‡1-888-723-3929LOVE IT OR IT'S ON US‡
Signature Care 44-045
-
INGREDIENTS AND APPEARANCE
PAIN RELIEVER
acetaminophen solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21130-945 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 1000 mg in 30 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21130-945-19 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 07/15/2021 Labeler - Better Living Brands, LLC (009137209) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 manufacture(21130-945) , pack(21130-945)