Label: ANTIMICROBIAL FOAM HANDWASH- chlorhexidine gluconate 2% solution liquid
- NDC Code(s): 21749-416-89, 21749-416-90
- Packager: GOJO Industries, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated August 24, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
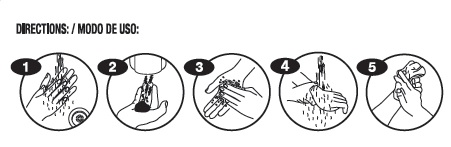
- Directions:/ Mood de Uso:
-
Warnings/Precauciones
For external use only
Allergy Alert:
This product may cause a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away
Para Use Externo Únicamente
Alerta De Allergia:
Este producto puede causar un reacción alérgica severa. Los síntomas pueden incluir:
- sibilancias/dificultad para respirar
- shock
- hinchazón facial
- urticaria
- sarpullido
En caso de ocurrir alguna reacción alérgica, detenga el uso y busque ayuda de inmediato
- Do not use/No debe usante
-
When using this product/ Cuando use este producto
Keep out of eyes, ear and mouth. May cause serious and permenant eye injury if placed or kept in the eye during surgical procedures or may cause deafness when instilled in the middle of the ear through perforated eardrums. If solution should contact these areas, rinse out promptly and thoroughly with water.
Evite el contacto con los ojos, oídos, y boca. Puede causar daño ocular serio y permanente si se coloca o permanece en el ojo durante procedimientos quirúrgicos o puede causar sordera si se instila en el oído medio a través de tímpano perforado. Si la solución llegara a tener contacto con estas àreas, enjuague lo más pronto posible con suficiente agua.
- Stop use an ask a doctor if/ Mantengase fuera consulte a su médico si
- Keep out of reach of children
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTIMICROBIAL FOAM HANDWASH
chlorhexidine gluconate 2% solution liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21749-416 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21749-416-89 1200 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2018 2 NDC:21749-416-90 1250 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019422 04/01/2018 Labeler - GOJO Industries, Inc. (004162038) Registrant - Xttrium Laboratories, Inc. (007470579) Establishment Name Address ID/FEI Business Operations Xttrium Laboratories, Inc. 007470579 manufacture(21749-416)