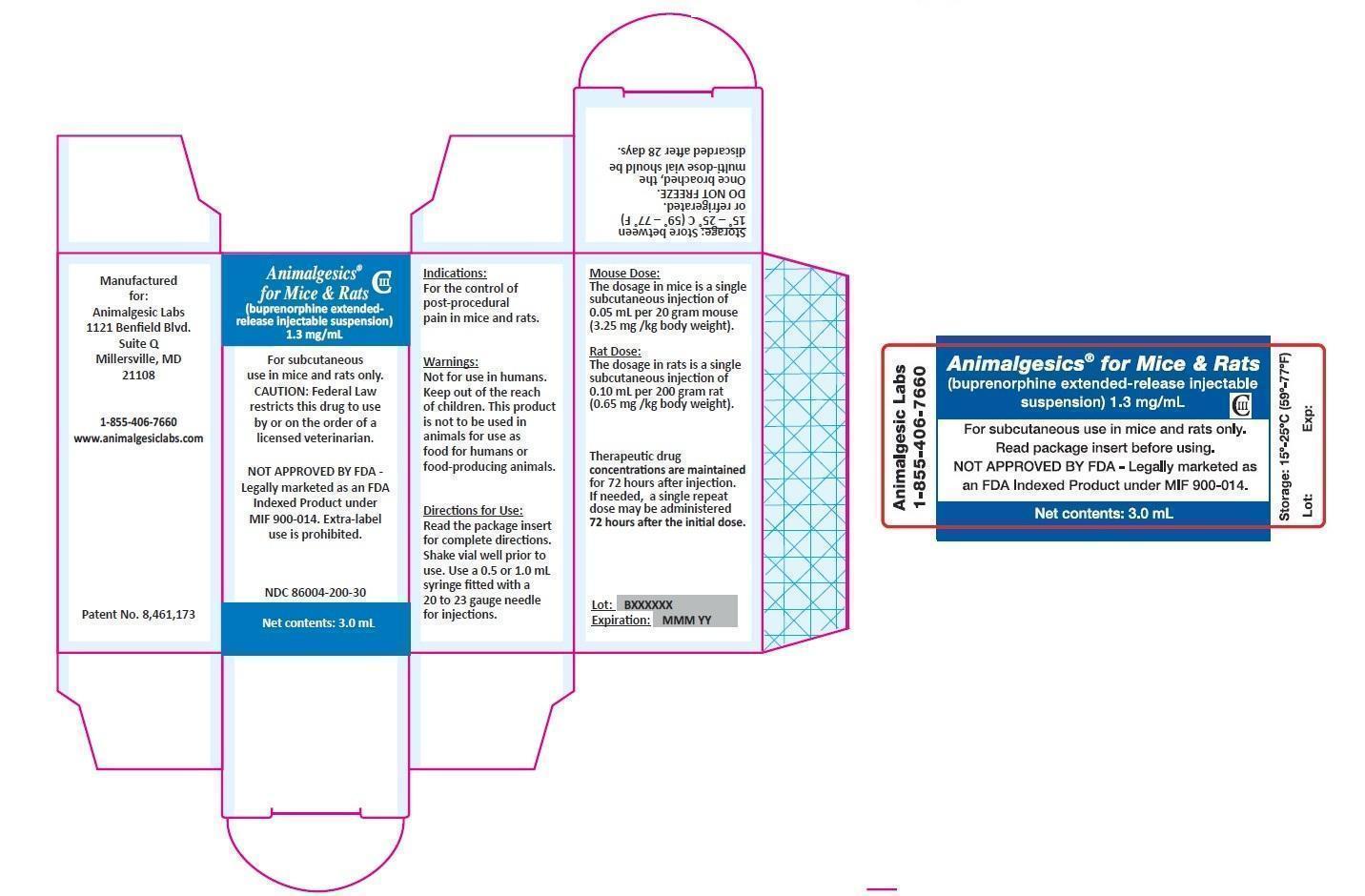
ANIMALGESICS FOR MICE AND RATS- buprenorphine hydrochloride injection, suspension, extended release
ANIMALGESIC LABORATORIES, INC.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Animalgesics for Mice & Rats
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
NOT APPROVED BY FDA – Legally Marketed as an FDA Indexed Product under MIF 900-014.
Extra-label Use is Prohibited.
Note – In order to be legally marketed, an animal drug product intended for a minor species must be Approved, Conditionally Approved, or Indexed by the FDA. THIS PRODUCT IS INDEXED.
For subcutaneous use in mice and rats only.
This product is not to be used in animals intended for use as food for humans or food-producing animals.
| WARNING: ABUSE POTENTIAL, LIFE-THREATENING RESPIRATORY DEPRESSION, and ACCIDENTAL EXPOSURE
Abuse Potential Animalgesics for Mice & Rats contains buprenorphine, a high concentration (1.3 mg/mL) opioid agonist and Schedule III controlled substance with an abuse potential similar to other Schedule III opioids. The high concentration of Animalgesics for Mice & Rats may be a particular target for human abuse. Buprenorphine has opioid properties that in humans may lead to dependence of the morphine type. Abuse of buprenorphine may lead to low or moderate physical dependence or high psychological dependence. The risk of abuse by humans should be considered when storing, administering, and disposing of Animalgesics for Mice & Rats. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (suicidal depression). Because of human safety risks, this drug should be used only with veterinary supervision. Do not dispense Animalgesics for Mice & Rats.
Life-Threatening Respiratory Depression
Animalgesics for Mice & Rats has additive CNS depressant effects when used with alcohol, other opioids, or illicit drugs that cause central nervous system depression. Because of the potential for adverse reactions associated with accidental injection, Animalgesics for Mice & Rats should only be administered by a veterinarian or laboratory staff trained in the handling of potent opioids. |
DESCRIPTION
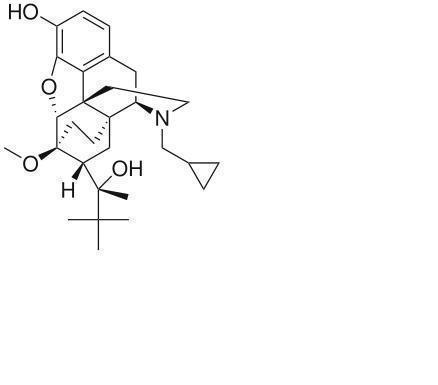
Animalgesics for Mice & Rats is an injectable suspension of extended-release buprenorphine. Buprenorphine hydrochloride, an opioid analgesic, is the active ingredient in Animalgesics for Mice & Rats. Lipid-bound buprenorphine hydrochloride is suspended in medium chain fatty acid triglyceride (MCT) oil. Lipids encapsulate the buprenorphine limiting diffusion which provides for larger doses and prolonged action.1,2Animalgesics for Mice & Rats has a slightly yellow to white opaque appearance. Each mL contains approximately 1.3 mg buprenorphine hydrochloride. The sterile product contains cholesterol, glyceryl tristearate, and buprenorphine hydrochloride suspended in MCT oil.
Buprenorphine
Formula C29H41NO4
INDICATIONS
Animalgesics for Mice & Rats is indicated for the control of post-procedural pain in mice and rats.
MOUSE DOSAGE AND ADMINISTRATION
Shake the vial briefly before each use to ensure uniform suspension. If stored refrigerated bring to room temperature before use.
Use aseptic technique to withdraw the dose in to a disposable 0.5 or 1.0 ml syringe. A 20 to 23 gauge needle should be used for injections due to the viscosity of the drug suspension.
The dosage of Animalgesics for Mice & Rats is a single subcutaneous injection of 0.05 mL per 20 gram mouse (3.25 mg/kg body weight). Therapeutic drug concentrations are maintained for 72 hours after the initial dose. If needed, a single repeat dose may be administered 72 hours after the initial dose.
Secure the mouse in a scruff-of-the-neck hold. Insert the needle into the dorsal subcutaneous space created by the scruff hold. Inject the entire dose into the dorsal subcutaneous space. An oily sheen may be observed in the dorsal fur of the mouse after injection due to leakage of the oil-based drug suspension from the injection site. The oily sheen may last for 4 to 5 days post-injection. Leakage from the injection site can be minimized by slowly injecting Animalgesics for Mice and Rats in to the subcutaneous space. The mouse can be returned to its cage immediately after receiving Animalgesics for Mice & Rats.
Do not return any unused drug suspension from the syringe back into the vial.
Once the vial is broached, Animalgesics for Mice & Rats can be stored at 15° to 25° C (59° – 77° F) or refrigerated for 28 days. DO NOT FREEZE.
RAT DOSAGE AND ADMINISTRATION
Shake the vial briefly before each use to ensure uniform suspension. If stored refrigerated bring to room temperature before use.
Use aseptic technique to withdraw the dose in to a disposable 0.5 or 1.0 ml syringe. A 20 to 23 gauge needle should be used for injections due to the viscosity of the drug suspension.
The dosage of Animalgesics for Mice & Rats is a single subcutaneous injection of 0.10 mL per 200 gram rat (0.65 mg/kg body weight). Therapeutic drug concentrations are maintained for 72 hours after the initial dose. If needed, a single repeat dose may be administered 72 hours after the initial dose.
Secure the rat in a passive restraint tube or by holding with a heavy glove with one person to secure the rat and a second person to administer the drug. Insert the needle into the dorsal subcutaneous space. Inject the entire dose into the dorsal subcutaneous space. An oily sheen may be observed in the dorsal fur after injection due to leakage of the oil-based drug suspension from the injection site. The oily sheen may last for 4 to 5 days post-injection. Leakage from the injection site can be minimized by slowly injecting Animalgesics for Mice and Rats in to the subcutaneous space. The rat can be returned to its cage immediately after receiving Animalgesics for Mice & Rats. See CONTRAINDICATIONS and Rat PRECAUTIONS for additional information on bedding.
Do not return any unused drug suspension from the syringe back into the vial.
Once the vial is broached, Animalgesics for Mice & Rats can be stored at 15° to 25° C (59° – 77° F) or refrigerated for 28 days. DO NOT FREEZE.
CONTRAINDICATIONS
Only administer Animalgesics for Mice & Rats by subcutaneous injection. Animalgesics for Mice & Rats is not intended for intravenous, intra-arterial, intrathecal, intramuscular, or intra-peritoneal injection.
Do not use on mice or rats with pre-existing respiratory deficiencies.
Do not keep rats on wood chip-type bedding after administration of Animalgesics for Mice & Rats.
HUMAN SAFETY WARNINGS
Not for use in humans. Keep out of the reach of children.
Adult Human User Safety while handling Animalgesics for Mice & Rats in the laboratory:
Two trained staff for administration: Animalgesics for Mice & Rats should only be handled and administered by laboratory staff trained in the handling of potent opioids. To prevent human adverse reactions or abuse, at least 2 trained administrators should be present during injection of Animalgesics for Mice & Rats.
Protective covering: To prevent direct contact of Animalgesics for Mice & Rats with human skin or mucous membranes when handling the suspension, protective clothing is recommended.
Mucous membrane or eye contact during administration: Direct contact of Animalgesics for Mice & Rats with the eyes, oral or other mucous membranes of humans could result in absorption of buprenorphine and the potential for adverse reactions. If accidental eye, oral or other mucous membrane contact is made during administration, flush the area with water and contact a physician.
Skin contact during administration: If human skin is accidentally exposed to Animalgesics for Mice & Rats, wash the exposed area with soap and water and contact a physician. Accidental exposure could result in absorption of buprenorphine and the potential for adverse reactions.
Drug Abuse, Addiction, and Diversion of Opioids:
Controlled Substance: Animalgesics for Mice & Rats contains buprenorphine, a mu opioid partial agonist and Schedule III controlled substance with an abuse potential similar to other Schedule III opioids. Animalgesics for Mice & Rats can be abused and is subject to misuse, abuse, addiction, and criminal diversion. Animalgesics for Mice & Rats should be handled appropriately to minimize the risk of diversion, including restriction of access, the use of accounting procedures, and proper disposal methods, as appropriate to the laboratory setting and as required by law.
Abuse: Abuse of Animalgesics for Mice & Rats poses a hazard of overdose and death. This risk is increased with concurrent abuse of alcohol and other substances including other opioids and benzodiazepines. Buprenorphine has been diverted for non-medical use into illicit channels of distribution. All people handling opioids require careful monitoring for signs of abuse. Drug abuse is the intentional non-therapeutic use of a prescription drug for its rewarding psychological or physiological effects. Abuse of opioids can occur in the absence of true addiction.
Storage and Discard: Animalgesics for Mice & Rats is a Class III opioid. Store in a locked, substantially constructed cabinet according to DEA and local controlled substance guidelines. Discard broached vials after 28 days. Any unused or expired vials must be destroyed by a DEA registered reverse distributor; for further information, call 1-855-406-7660.
Physician information: Animalgesics for Mice & Rats injectable suspension is a mu opioid partial agonist (1.3 mg buprenorphine/mL). In the case of an emergency, provide the physician with the package insert. Naloxone may not be effective in reversing respiratory depression produced by buprenorphine. The onset of naloxone effect may be delayed by 30 minutes or more. Doxapram hydrochloride has also been used as a respiratory stimulant.
PRECAUTIONS
Mice
The safety of Animalgesics for Mice & Rats has not been evaluated in pregnant, lactating, neonatal, or immune-compromised mice. As with other opioids, buprenorphine may cause sedation, decreased blood pressure, decreased heart rate, decreased gastrointestinal mobility, and respiratory depression. Use caution with concomitant administration of Animalgesics for Mice & Rats with drugs that cause respiratory depression. The use of paper or soft bedding for up to 3 days following administration of Animalgesics for Mice & Rats should be considered.
Normal mice may exhibit an obtunded response to stimuli up to 4 hours after receiving Animalgesics for Mice & Rats.
Buprenorphine is excreted in the feces (see Clinical Pharmacology section below). Coprophagy may lead to ingestion of buprenorphine or its metabolites by mice treated with Animalgesics for Mice & Rats and untreated cage mates.
Rats
The safety of Animalgesics for Mice & Rats has not been evaluated in pregnant, lactating, neonatal, or immune-compromised rats. As with other opioids, buprenorphine may cause sedation, decreased blood pressure, decreased heart rate, decreased gastrointestinal mobility, and respiratory depression. Use caution with concomitant administration of Animalgesics for Mice & Rats with drugs that cause respiratory depression.
Rats may exhibit signs of nausea including Pica up to 3 days post-treatment. Rats should be maintained on paper or soft bedding to avoid ingestion of wood chip-type bedding after administration of Animalgesics for Mice & Rats. Pica involving wood chip-type bedding can be lethal in rats.
Buprenorphine is excreted in the feces (see Clinical Pharmacology section below). Coprophagy may lead to ingestion of buprenorphine or its metabolites by rats treated with Animalgesics for Mice & Rats and untreated cage mates.
ADVERSE REACTIONS
For technical assistance, or to report an adverse drug reaction, please call Animalgesic Laboratories at 1-855-406-7660.
Adverse drug reactions may also be reported to the FDA/CVM at 1-888-FDA-VETS or http://www.fda.gov/AnimalVeterinary/SafetyHealth.
Mice
No adverse reactions were observed in 20 to 25 gram young adult male and female mice after a single subcutaneous injection of Animalgesics for Mice & Rats at a dose 5 times the indicated dose. Laboratory parameters evaluated in the study included hematology and clinical chemistry; histopathology was also performed. In a second study, adult male and female mice received Animalgesics for Mice & Rats subcutaneously at 5 times the indicated dose for three doses at four day intervals. A surgical procedure was performed on the study mice prior to receiving each of the three doses of Animalgesics for Mice & Rats.
Mortality was seen in two male mice after the third surgical procedure and dose of Animalgesics for Mice & Rats (total dose of 49 mg buprenorphine/kg body weight in 8 days).
Weight loss has been observed in mice treated post-procedurally with Animalgesics for Mice & Rats.
Rats
Adverse reactions were evaluated in 180 to 200 gram young adult male and female rats after a single injection of Animalgesics for Mice & Rats. A surgical procedure was performed on the rats prior to administration of a single dose at the intended dose of 0.65 mg/kg or a single dose of 2, 6 or 10-fold excess dose. Adverse reactions also were evaluated in male and female mice administered 2, 6 and 10 times the intended dose for three doses at four day intervals. A surgical procedure was performed on the rats prior to administration of the first of three doses. Laboratory parameters evaluated in the study included hematology, clinical chemistry, urinalysis, histopathology, and bodyweight.
Signs of nausea were observed at all dose levels within 24 hours of the dose. Signs included self-licking, self-gnawing and efforts to eat wood-chip bedding.
Mortality was seen in 1 of 36 rats exposed to wood chip bedding. Necropsy revealed the stomach and esophagus were compacted with bedding, the bladder was abnormally distended and the urine contained blood.
Mortality was seen in 3 of 222 rats treated with Animalgesics for Mice & Rats due to technical complications with serial bleeding of the jugular vein.
CLINICAL PHARMACOLOGY3
Buprenorphine can act as an agonist and antagonist at different classes of opioid receptors. Agonism at the mu opioid receptor and, in some cases, antagonism at the kappa or delta opioid receptors are possible underlying mechanisms for the ceiling effect and bell-shaped dose-response curve of buprenorphine. Studies with knockout mice have shown that the antinociceptive effect of buprenorphine, which is mediated primarily by the mu opioid receptor, is attenuated by the ability of the drug to activate the opioid receptor like (ORL-1) receptor. The drug can be described as a ‘full’ and a ‘partial’ agonist at the same receptor depending on the specific assay. There appears to be no ceiling effect for analgesia, but there is a ceiling effect for respiratory depression.
Pharmacokinetic studies with bolus injections of buprenorphine in mice and rats provide similar models. After bolus intravenous administration, plasma levels decline tri-exponentially. The drug is n-deakylated in the liver to norbuprenorphine (NBN), an active metabolite. Studies have shown that glucuronide metabolites of buprenorphine and NBN are also metabolically active, and can approximate or exceed the concentration of the parent drug. Un-metabolized drug excreted in the urine and feces one week after injection was 1.9 and 22.4% of the dose, respectively, and 92% of the dose was accounted for in one week3
Mice
Pharmacokinetic parameters of Animalgesics for Mice & Rats were studied in 6-8 week old male and female Balb/c mice following a single subcutaneous injection of 3.25 mg/kg bodyweight. Clinically significant blood levels were observed up to 72 hours after subcutaneous injection.
Rats
Pharmacokinetic parameters of Animalgesics for Mice & Rats were studied in 8 week old male and female Fischer rats following a single subcutaneous injection of 0.65 mg/kg bodyweight. Clinically significant blood levels were observed up to 72 hours after subcutaneous injection.
STORAGE INFORMATION
Store between 15° and 25° C (59° – 77° F) or refrigerated. DO NOT FREEZE. If stored refrigerated, bring to room temperature before use. Once broached, the multi-dose vial should be discarded after 28 days.
REFERENCES
1. Mishra et al., Drug Delivery and Transl. Res, 2:238-253; 2012.
2. Bethuneet al., The role of drug-lipid interactions on the disposition of liposome-formulated opioid analgesics in vitro and in vivo.AnesthAnalg. 93(4):928-33; 2001.
3. Guarnieri et al, Lab Animal, 41(11): 337-343; 2012.
HOW SUPPLIED
Animalgesics for Mice & Rats is supplied in a multi-use glass vial containing 3.0 mL or 1.6 mL of injectable drug suspension.
| Animalgesics for Mice & Rats | 1.6 mL vial | NDC 86004-200-16 |
| Animalgesics for Mice & Rats | 3.0 mL vial | NDC 86004-200-30 |
| ANIMALGESICS FOR MICE AND RATS
buprenorphine hydrochloride injection, suspension, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - ANIMALGESIC LABORATORIES, INC. (788213093) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Noramco, Inc. | 057234486 | api manufacture | |