Label: NIGHTTIME COLD AND FLU MULTI SYMPTOM- acetaminophen, dextromethorphan hydrobromide, doxylamine succinate capsule, liquid filled
- NDC Code(s): 62011-0389-1
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 27, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
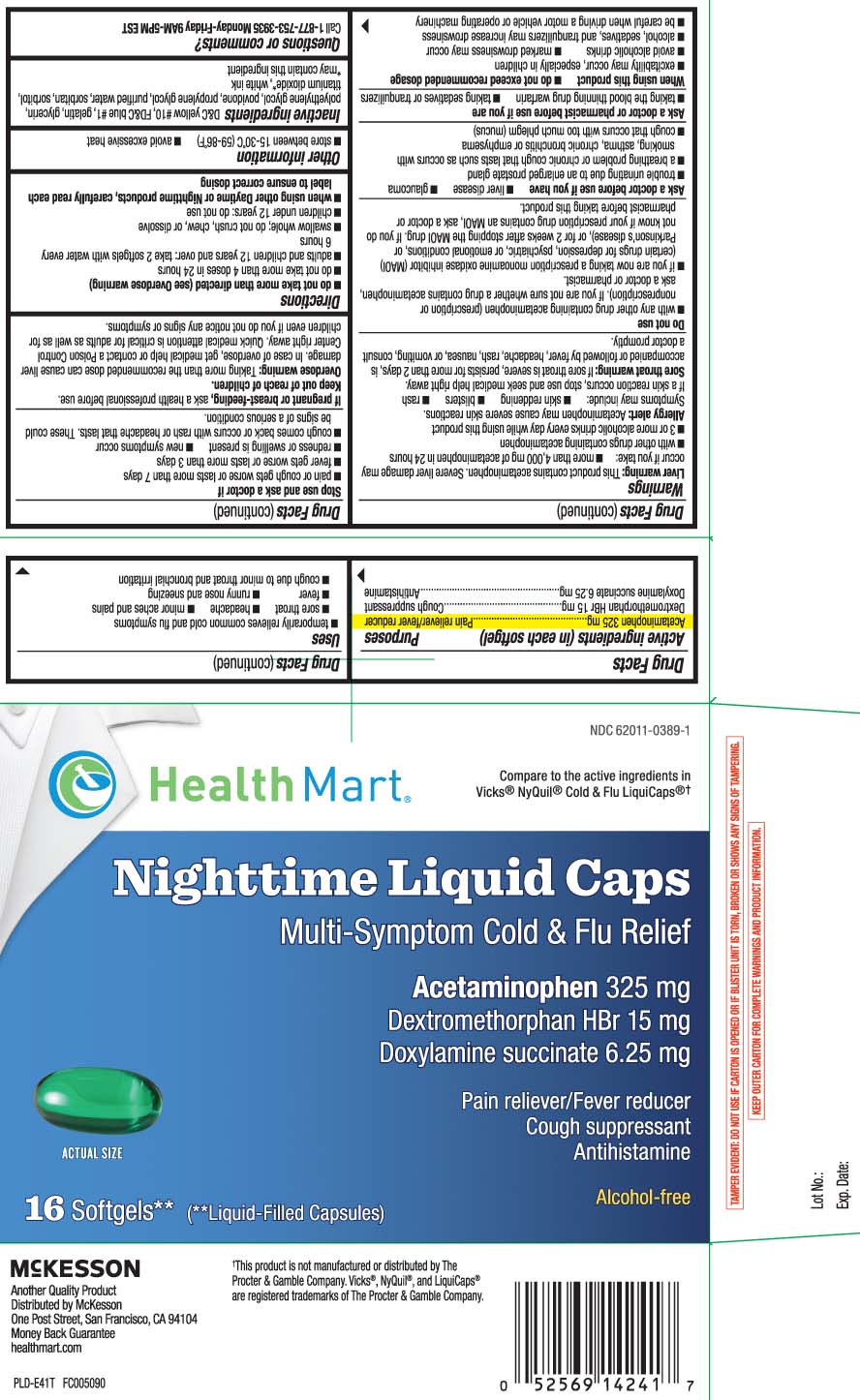
- Active Ingredients (in each softgel)
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- Skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor of pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough that occurs with too much phlegm (mucus)
Ask a doctor of pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- do not exceed recommended dosage
- excitability may occur, especially in children
- avoid alcoholic drinks
- marked drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- pain or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose can cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
-
Directions
- do not take more than directed (see Overdose warning)
- do not take more than 4 doses in 24 hours
- adults and children 12 years and over: take 2 softgels with water every 6 hours
- swallow whole; do not crush, chew, or dissolve
- children under 12 years: do not use
- when using other Daytime or Nighttime products, carefully read each label to insure correct dosing
- Other Information
- Inactive Ingredients
- Questions or Comments?
-
Principal Display Panel
Compare to the active ingredients in Vicks® NyQuil® Cold & Flu LiquiCaps®†
Nighttime Liquid Caps
Multi-Symptom Cold & Flu Relief
Acetaminophen 325 mg
Dextromethorphan HBr 15 mg
Doxylamine Succinate 6.25 mg
Pain reliever / Fever reducer
Cough suppressant
Antihistamine
Alcohol-free
Softgels** (**Liquid-Filled Capsules)
†This product is not manufactured or distributed by The Procter & Gamble Company. Vicks®, NyQuil®, and LiquiCaps® are registered trademarks of The Procter & Gamble Company.
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Another Quality Product
Distributed by McKesson
One Post Street, San Francisco, CA 94104
- Package Label
-
INGREDIENTS AND APPEARANCE
NIGHTTIME COLD AND FLU MULTI SYMPTOM
acetaminophen, dextromethorphan hydrobromide, doxylamine succinate capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62011-0389 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 15 mg DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 6.25 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITAN (UNII: 6O92ICV9RU) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color green Score no score Shape OVAL Size 20mm Flavor Imprint Code P30;94A;AP017 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62011-0389-1 16 in 1 CARTON 09/30/2018 05/31/2024 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 09/30/2018 05/31/2024 Labeler - Strategic Sourcing Services LLC (116956644)