C-M-P-K- calcium-magnesium -phosphorous-potassium-dextrose injection, solution
VetTek
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
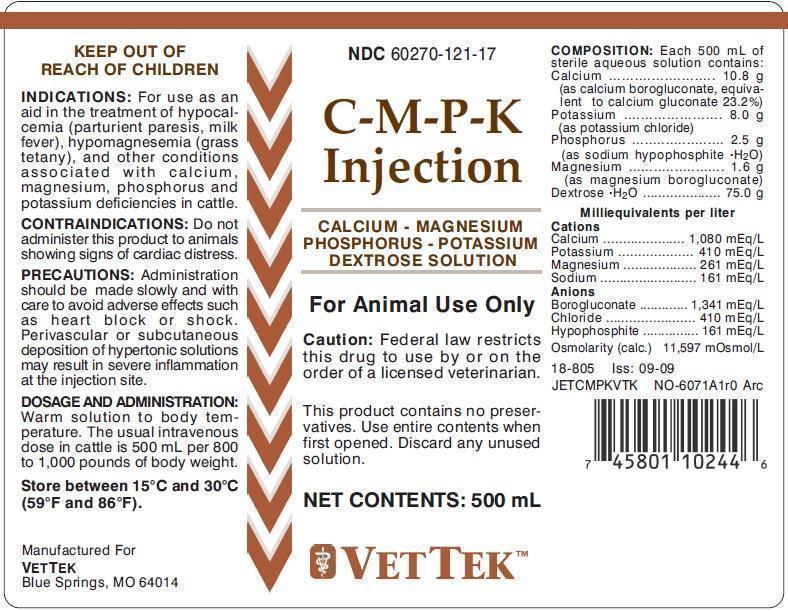
C-M-P-K Injection
INDICATIONS
For use as an aid in the treatment of hypocalcemia (parturient paresis, milk fever), hypomagnesemia (grass tetany), and other conditions associated with calcium, magnesium, phosphorous and potassium deficencies in cattle.
CONTRAINDICATIONS
DO NOT administer this product to animals showing signs of cardiac distress. Monitor animal's condition closely.
PRECAUTIONS
Administration should be made slowly and with care to avoid adverse effects such as heart block or shock. Perivascular or subcutaneous deposition of hypertonic solutions may result in severe inflammation at the injection site.
DOSAGE & ADMINISTRATION
Warm solution to body temperature. The usual intravenous dose in cattle is 500 mL per 800 to 1,000 pounds of body weight.
EACH 500 ML CONTAINS:
Calcium..........................10.8 g
(as calcium borogluconate, equivalent to calcium gluconate 23.2%)
Potassium......................8.0 g
(as potassium chloride)
Phosphorus..................2.5 g
(as sodium hypophosphite - H2O)
Magnesium..................1.6 g
(as magnesium borogluconate)
Dextrose - H2O.............75.0 g
Milliequivalents per liter
Cations
Calcium....................1,080 mEq/L
Potassium..................410 mEq/L
Magnesium...............410 mEq/L
Sodium.......................410 mEq/L
Anions
Borogluconate.........1,341 mEq/L
Chloride.......................410 mEq/L
Hypophosphite..........161 mEq/L
Osmolarity (calc.) 11,597 mOsmol/L
| C-M-P-K
calcium-magnesium -phosphorous-potassium-dextrose injection, solution |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - VetTek (056387798) |
| Registrant - VetTek (056387798) |