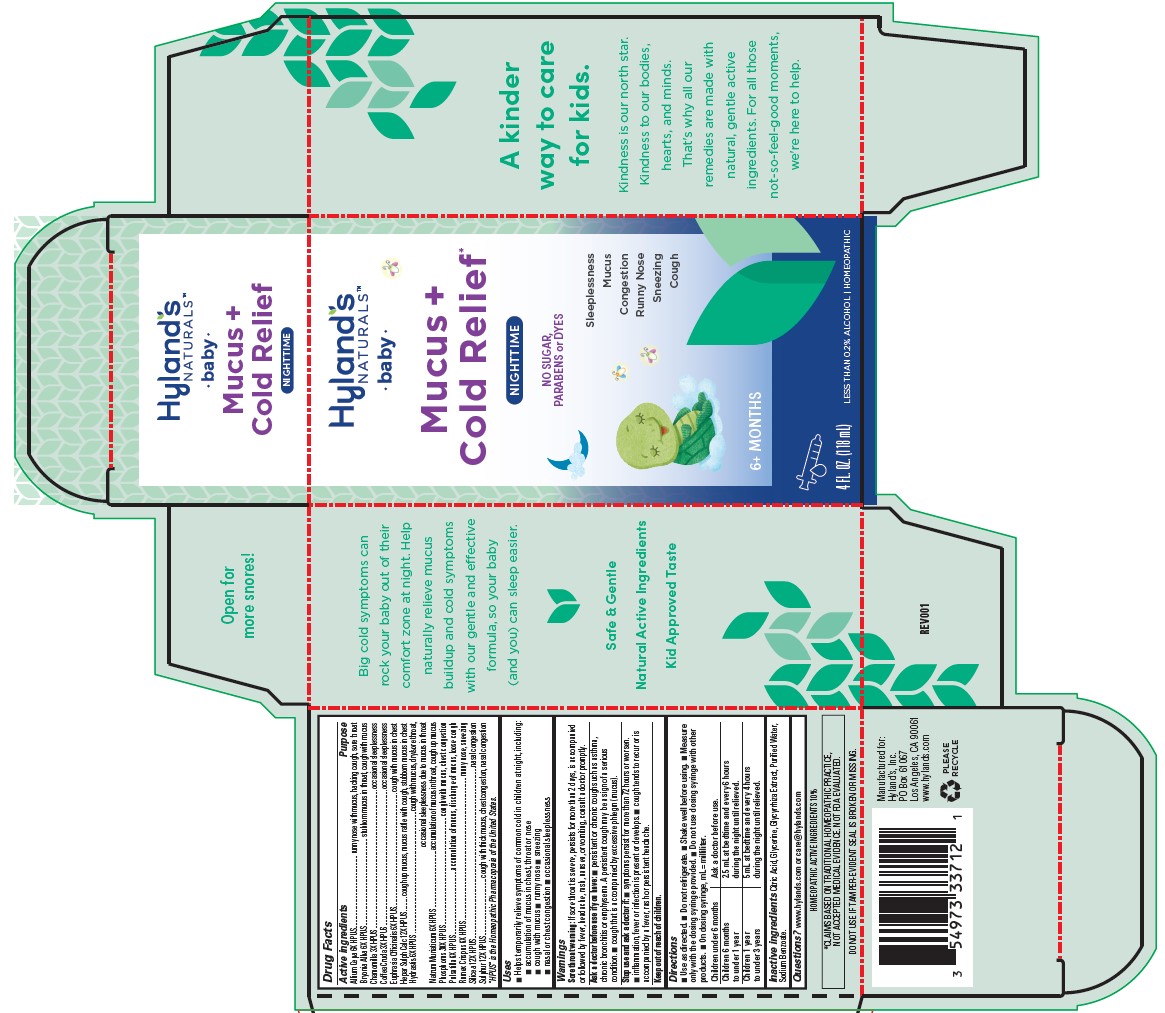
Label: BABY NIGHTTIME MUCUS PLUS COLD RELIEF- calcium sulfide,phosphorus,sulfur,rumex crispus root,sodium chloride,arabica coffee bean,anemone pulsatilla,silicon dioxide, goldenseal,onion,bryonia alba root,euphrasia stricta and matricaria chamomilla liquid
- NDC Code(s): 54973-3371-1, 54973-3371-2, 54973-3371-3
- Packager: Hyland's Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 17, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Purpose
-
Drug Facts
Active ingredients
Purpose
Allium Cepa 6X HPUS
runny nose with mucus, hacking cough, sore throat
Bryonia Alba 6X HPUS
stubborn mucus in throat, cough with mucus
Chamomilla 3X HPUS
occasional sleeplessness
Coffea Cruda 3X HPUS
occasional sleeplessness
Euphrasia Officinalis 6X HPUS
cough with mucus in chest
Hepar Sulph Calc 12X HPUS
cough up mucus, mucus rattle with cough, stubborn mucus in chest
Hydrastis 6X HPUS
cough with mucus, dry/sore throat, occasional sleeplessness due to mucus in throat
Natrum Muriaticum 6X HPUS
accumulation of mucus in throat, cough up mucus
Phosphorus 30X HPUS
cough with mucus, chest congestion
Pulsatilla 6X HPUS
accumulation of mucus, discharge of mucus, loose cough
Rumex Crispus 6X HPUS
runny nose, sneezing
Silicea 12X HPUS
nasal congestion
Sulfur 12X HPUS
cough with thick mucus, chest congestion, nasal congestion
- Uses
-
Warnings
Sore throat warning:
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Ask a doctor before use if you have:
■ persistent or chronic cough such as asthma, chronic bronchitis or emphysema. A persistent cough may be a sign of a serious condition. ■ cough that is accompanied by excessive phlegm (mucus).
-
Directions
■ Use as directed. ■ Do not refrigerate. ■ Shake well before using. ■ Measure only with the dosing syringe provided. ■ Do not use dosing syringe with other products. ■ On dosing syringe, mL = milliliter.
Children under 6 months Ask a doctor before use. Children 6 months
to under 1 year2.5 mL at bedtime and every 6 hours
during the night until relieved.Children 1 year
to under 3 years5 mL at bedtime and every 4 hours
during the night until relieved. - Inactive ingredients
- Questions?
- *CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
- DO NOT USE IF TAMPER-EVIDENT SEAL IS BROKEN OR MISSING.
- HOMEOPATHIC ACTIVE INGREDIENTS 10%
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BABY NIGHTTIME MUCUS PLUS COLD RELIEF
calcium sulfide,phosphorus,sulfur,rumex crispus root,sodium chloride,arabica coffee bean,anemone pulsatilla,silicon dioxide, goldenseal,onion,bryonia alba root,euphrasia stricta and matricaria chamomilla liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54973-3371 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 12 [hp_X] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_X] in 1 mL ANEMONE PULSATILLA (UNII: I76KB35JEV) (ANEMONE PULSATILLA - UNII:I76KB35JEV) ANEMONE PULSATILLA 6 [hp_X] in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 6 [hp_X] in 1 mL ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 6 [hp_X] in 1 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 6 [hp_X] in 1 mL EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 6 [hp_X] in 1 mL MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA 3 [hp_X] in 1 mL ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 3 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 6 [hp_X] in 1 mL RUMEX CRISPUS ROOT (UNII: 9N1RM2S62C) (RUMEX CRISPUS ROOT - UNII:9N1RM2S62C) RUMEX CRISPUS ROOT 6 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54973-3371-1 1 in 1 CARTON 11/01/2018 12/27/2021 1 148 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:54973-3371-2 1 in 1 CARTON 11/01/2018 2 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC:54973-3371-3 2.5 mL in 1 PACKET; Type 0: Not a Combination Product 11/01/2018 12/27/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/01/2018 Labeler - Hyland's Inc. (008316655) Establishment Name Address ID/FEI Business Operations Hyland's Inc. 008316655 manufacture(54973-3371) , pack(54973-3371) , label(54973-3371)