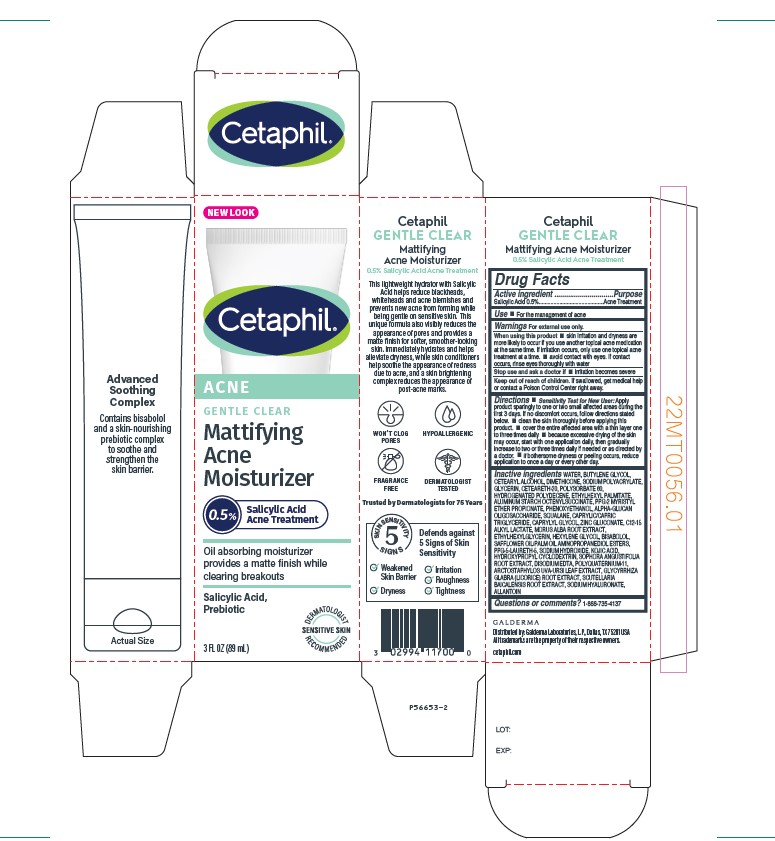
Label: CETAPHIL GENTLE CLEAR MATTIFYING ACNE MOISTURIZER- salicylic acid lotion
- NDC Code(s): 0299-4117-00, 0299-4117-05
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Use
-
Warnings
For external use only.
When using this product • skin irritation and dryness are more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne treatment at a time.• avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if ● irritation becomes severe.
- Keep out of reach of children.
-
Directions
•Sensitivity Test for New User:
Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow directions stated below. • clean the skin thoroughly before applying this product. • cover the entire affected area with a thin layer one to three times daily • because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor. • if bothersome dryness or peeling occurs, reduce application to once a day or every other day
-
Inactive Ingredients
Water, Butylene Glycol, Cetearyl Alcohol, Dimethicone, Sodium Polyacrylate, Glycerin, Ceteareth-20, Polysorbate 60, Hydrogenated Polydecene, Ethylhexyl Palmitate, Aluminum Starch Octenylsuccinate, PPG-2 Myristyl Ether Propionate, Phenoxyethanol, Alpha-Glucan Oligosaccharide, Squalane, Caprylic/Capric Triglyceride, Caprylyl Glycol, Zinc Gluconate, C12-15 Alkyl Lactate, Morus Alba Root Extract, Ethylhexylglycerin, Hexylene Glycol, Bisabolol, Safflower Oil/Palm Oil Aminopropanediol Esters, PPG-5-Laureth-5, Sodium Hydroxide, Kojic Acid, Hydroxypropyl Cyclodextrin, Sophora Angustifolia Root Extract, Disodium EDTA, Polyquaternium-11, Arctostaphylos Uva-Ursi Leaf Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Scutellaria Baicalensis Root Extract, Sodium Hyaluronate, Allantoin
- Questions or comments?
-
PRINCIPLE DISPLAY PANEL - 3 FL OZ Carton
NEW LOOK
Cetaphil®
ACNE
GENTLE CLEARMattifying
Acne
Moisturizer
0.5% Salicylic Acid Acne Treatment
Oil Absorbing moisturizer
provides a matte finish while
clearing breakouts
Salicylic Acid,
Prebiotic
Dermatologist Recommended
Sensitive Skin
3 FL OZ (89 mL)
Distributed by: Galderma Laboratories, L.P., Dallas TX 75201USA
All trademarks are the property of their respective owners.
cetaphil.com
P56653-2
-
INGREDIENTS AND APPEARANCE
CETAPHIL GENTLE CLEAR MATTIFYING ACNE MOISTURIZER
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0299-4117 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Butylene Glycol (UNII: 3XUS85K0RA) Cetostearyl Alcohol (UNII: 2DMT128M1S) Dimethicone (UNII: 92RU3N3Y1O) Sodium Polyacrylate (2500000 Mw) (UNII: 05I15JNI2J) Glycerin (UNII: PDC6A3C0OX) Polyoxyl 20 Cetostearyl Ether (UNII: YRC528SWUY) Polysorbate 60 (UNII: CAL22UVI4M) Hydrogenated Polydecene (550 Mw) (UNII: U333RI6EB7) Ethylhexyl Palmitate (UNII: 2865993309) Aluminum Starch Octenylsuccinate (UNII: I9PJ0O6294) Ppg-2 Myristyl Ether Propionate (UNII: 88R97D8U8A) Phenoxyethanol (UNII: HIE492ZZ3T) .Alpha.-Glucan Oligosaccharide (UNII: S95658MI3W) Squalane (UNII: GW89575KF9) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Caprylyl Glycol (UNII: 00YIU5438U) Zinc Gluconate (UNII: U6WSN5SQ1Z) C12-15 Alkyl Lactate (UNII: GC844VRD7E) Morus Alba Root (UNII: CST1G9BZGD) Ethylhexylglycerin (UNII: 147D247K3P) Hexylene Glycol (UNII: KEH0A3F75J) Levomenol (UNII: 24WE03BX2T) Safflower Oil (UNII: 65UEH262IS) Ppg-25-Laureth-25 (UNII: 2R54TS98QU) Sodium Hydroxide (UNII: 55X04QC32I) Kojic Acid (UNII: 6K23F1TT52) Hydroxypropyl Betadex (UNII: 1I96OHX6EK) Sophora Flavescens Root (UNII: IYR6K8KQ5K) Edetate Disodium (UNII: 7FLD91C86K) Polyquaternium-11 (1000000 Mw) (UNII: 0B44BS5IJS) Arctostaphylos Uva-Ursi Leaf (UNII: 3M5V3D1X36) Glycyrrhiza Glabra (UNII: 2788Z9758H) Scutellaria Baicalensis Root (UNII: 7J95K7ID2S) Hyaluronate Sodium (UNII: YSE9PPT4TH) Allantoin (UNII: 344S277G0Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-4117-00 1 in 1 CARTON 05/01/2021 1 89 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0299-4117-05 5 mL in 1 TUBE; Type 0: Not a Combination Product 05/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 05/01/2021 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations Cosmetic Essence, LLC dba Voyant Beauty 032565959 manufacture(0299-4117)