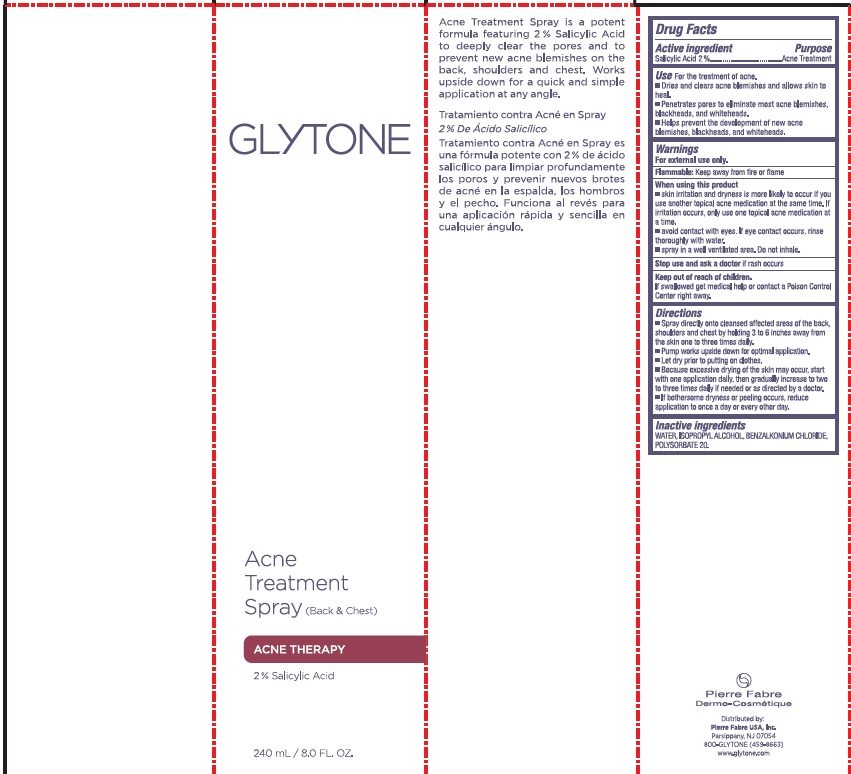
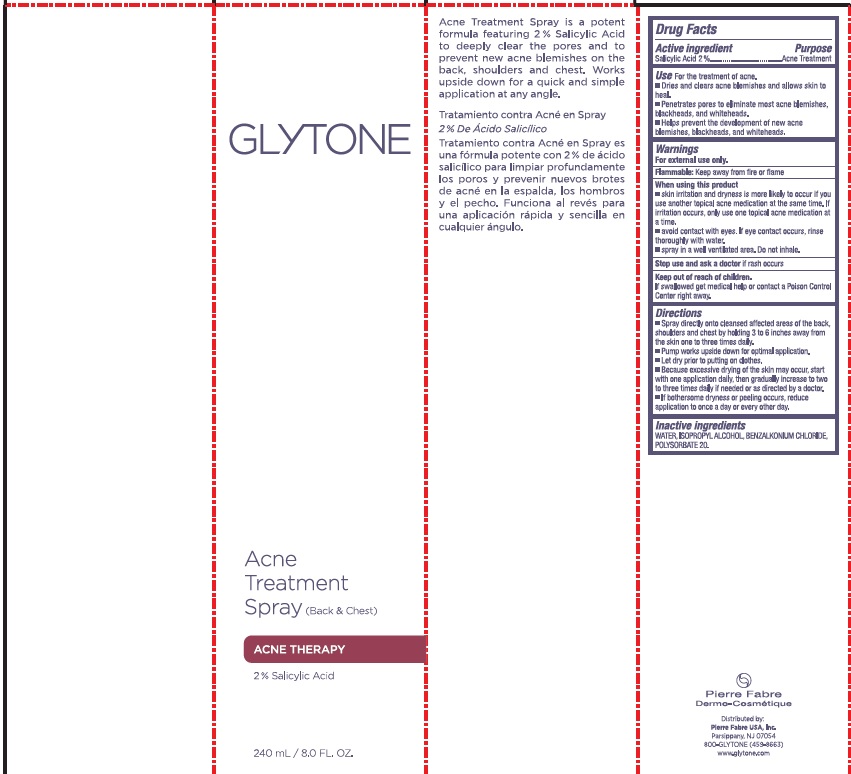
Label: GLYTONE ACNE BACK AND CHEST TREATMENT- salicylic acid spray
GLYTONE ACNE TREATMENT- salicylic acid spray
- NDC Code(s): 64760-711-01, 64760-711-02, 64760-735-01
- Packager: Pierre Fabre USA Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Use
-
Warnings
When using this product
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs use only one topical acne medication at a time.
- Avoid contact with eyes. If eye contact occurs, rinse thoroughly with water.
- Spray in a well ventilated area. Do not inhale.
-
Directions
Spray directly onto cleansed affected areas of the back, shoulders and chest by holding 3 to 6 inches away from the skin one to three times daily.
Pump works upside down and for optimal application.
Let dry prior to putting on clothes.
Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two to three times daily if needed or as directed by a doctor.
-
Inactive Ingredients
AQUA (WATER), ALCOHOL DENAT., METHYLPROPANEDIOL, GLUCONOLACTONE, POLYSORBATE 20, POTASSIUM SORBATE, SODIUM GLUCONATE, SODIUM BENZOATE, CITRIC ACID, GLYCERIN, HIBISCUS SARBARIFFA FLOWER EXTRACT, CENTELLA ASIATICA (GOTU KOLA) LEAF EXTRACT, CAMELLIA SINENSIS (WHITE TEA) LEAF EXTRACT, CAMELLIA OLEIFERA (GREEN TEA) LEAF EXTRACT, ALOE BARBADENSIS LEAF JUICE POWDER, SODIUM HYDROXIDE
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GLYTONE ACNE BACK AND CHEST TREATMENT
salicylic acid sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64760-735 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength HIBISCUS SABDARIFFA FLOWER (UNII: 45TGG6IU6M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CENTELLA ASIATICA (UNII: 7M867G6T1U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) SODIUM HYDROXIDE (UNII: 55X04QC32I) CALCIUM GLUCONATE (UNII: SQE6VB453K) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM GLUCONATE (UNII: R6Q3791S76) GLUCONOLACTONE (UNII: WQ29KQ9POT) ALCOHOL (UNII: 3K9958V90M) SODIUM BENZOATE (UNII: OJ245FE5EU) METHYLPROPANEDIOL (UNII: N8F53B3R4R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64760-735-01 1 in 1 CARTON 09/01/2021 1 240 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 09/01/2021 GLYTONE ACNE TREATMENT
salicylic acid sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64760-711 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) POLYSORBATE 20 (UNII: 7T1F30V5YH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64760-711-01 240 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 03/06/2018 06/22/2023 2 NDC:64760-711-02 60 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 03/06/2018 06/22/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 03/06/2018 06/22/2023 Labeler - Pierre Fabre USA Inc. (117196928) Registrant - Pierre Fabre USA Inc. (117196928)