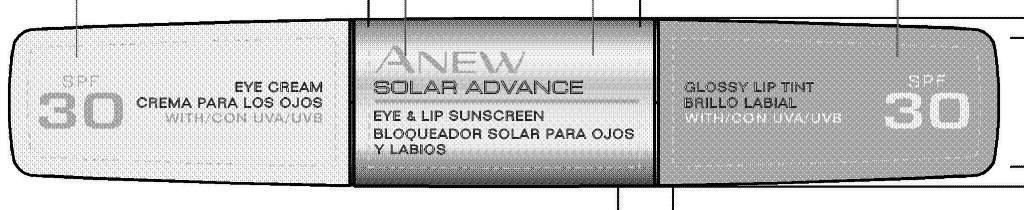
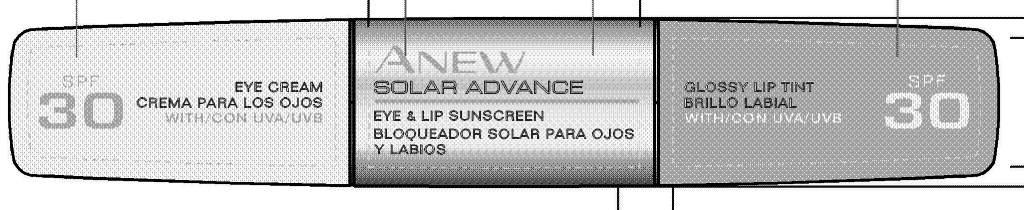
Label: ANEW SOLAR ADVANCE EYE AND LIP SUNSCREEN- titanium dioxide, zinc oxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 10096-0241-1, 10096-0241-2, 10096-0241-3 - Packager: Avon Products, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 21, 2010
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
-
ACTIVE INGREDIENT
EYE CREAM ACTIVE
INGREDIENTS/INGREDIENTES
ACTIVOS DE LA
CREMA PARA LOS OJOS:
TITANIUM DIOXIDE 3.1%,
ZINC OXIDE 6.9%.OTHER INGREDIENTS/
OTROS INGREDIENTES:
WATER/EAU, DIMETHICONE,
GLYCERIN, SIMMONDSIA
CHINENSIS (JOJOBA) SEED
OIL, DIMETHICONE CROSSPOLYMER,
PROPYLENE
GLYCOL, ISOCETYL STEAROYL
STEARATE, ISODECYL
ISONONANOATE, PHENYL
TRIMETHICONE, MAGNESIUM
SULFATE, VP/HEXADECENE
COPOLYMER, PEG-10
DIMETHICONE, LAURYL
PEG-9 POLYDIMETHYLSILOXYETHYL
DIMETHICONE,
THIODIPROPIONIC ACID, GLYCINE
SOJA (SOYBEAN) SEED
EXTRACT, PHAEODACTYLUM
TRICORNUTUM EXTRACT,
ORYZANOL, FOENICULUM
VULGARE (FENNEL) FRUIT EXTRACT,
DAUCUS CAROTA
SATIVA (CARROT) ROOT EXTRACT,
GLYCINE MAX (SOYBEAN)
SYMBIOSOME EXTRACT,
TOCOPHEROL, TOCOPHERYL
ACETATE, POLYESTER-
8, CAPRYLIC/CAPRIC
TRIGLYCERIDE, SILICA, HYDRATED
SILICA, POLYMETHYL
METHACRYLATE, ALUMINUM
HYDROXIDE, DIMETHICONE/
METHICONE COPOLYMER,
DIMETHICONE/DIVINYLDIMETHICONE/
SILSESQUIOXANE
CROSSPOLYMER,POLYSORBATE 20, POLYSORBATE
80, GLYCERYL STEARATE,
POLYHYDROXYSTEARIC
ACID, PEG-30 DIPOLYHYDROXYSTEARATE,
TRIETHOXYCAPRYLYLSILANE,
ISOHEXADECANE, AMMONIUM
POLYACRYLOYLDIMETHYL
TAURATE, AMMONIUM
HYDROXIDE, CAPRYLYL
GLYCOL, METHYLPARABEN,
IMIDAZOLIDINYL UREA,
DISODIUM EDTA. -
ACTIVE INGREDIENT
GLOSSY LIP TINT
ACTIVE INGREDIENTS/
INGREDIENTES ACTIVOS
DEL BRILLO LABIAL: TITANIUM
DIOXIDE 3.3%,
ZINC OXIDE 7.2%.OTHER INGREDIENTS/
OTROS INGREDIENTES:
POLYBUTENE, BIS-DIGLYCERYL
POLYACYLADIPATE-2,
C18-36 ACID TRIGLYCERIDE,
SIMMONDSIA CHINENSIS
(JOJOBA) SEED OIL, PHENYL
TRIMETHICONE, ISOCETYL
STEAROYL STEARATE, ISODECYL
ISONONANOATE, DIISOSTEARYL
FUMARATE,
KAEMPFERIA GALANGA
ROOT EXTRACT, GLYCINE
SOJA (SOYBEAN) SEED EXTRACT,
PHAEODACTYLUM
TRICORNUTUM EXTRACT,
ORYZANOL, FOENICULUM
VULGARE (FENNEL) FRUIT EXTRACT,
DAUCUS CAROTA
SATIVA (CARROT) ROOT EXTRACT,
DILAURYL THIODIPROPIONATE,
TOCOPHEROL,
GLYCERIN, WATER/EAU,
POLYESTER-8, POLYHYDROXYSTEARIC
ACID, GLYCERYL
STEARATE, HYDRATED
SILICA, CAPRYLIC/CAPRIC
TRIGLYCERIDE, DIMETHICONE/
METHICONE COPOLYMER,
ALUMINUM HYDROXIDE,
TRIETHOXYCAPRYLYLSILANE,
CAPRYLYL GLYCOL,
PARFUM/FRAGRANCE, IRON
OXIDES, MICA/CI 77019,
CARMINE/CI 75470. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANEW SOLAR ADVANCE EYE AND LIP SUNSCREEN
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10096-0241 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.155 g in 5 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.345 g in 5 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.165 g in 5 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.36 g in 5 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) HYDRATED SILICA (UNII: Y6O7T4G8P9) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYSORBATE 20 (UNII: 7T1F30V5YH) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PEG-30 DIPOLYHYDROXYSTEARATE (UNII: 9713Q0S7FO) ISOHEXADECANE (UNII: 918X1OUF1E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) METHYLPARABEN (UNII: A2I8C7HI9T) C18-36 ACID TRIGLYCERIDE (UNII: ZRA72DR3R7) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10096-0241-3 1 in 1 CARTON 1 NDC:10096-0241-1 5 g in 1 TUBE, WITH APPLICATOR 2 NDC:10096-0241-3 1 in 1 CARTON 2 NDC:10096-0241-2 5 g in 1 TUBE, WITH APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/21/2010 Labeler - Avon Products, Inc. (001468693) Establishment Name Address ID/FEI Business Operations Avon Products, Inc. 005149471 manufacture