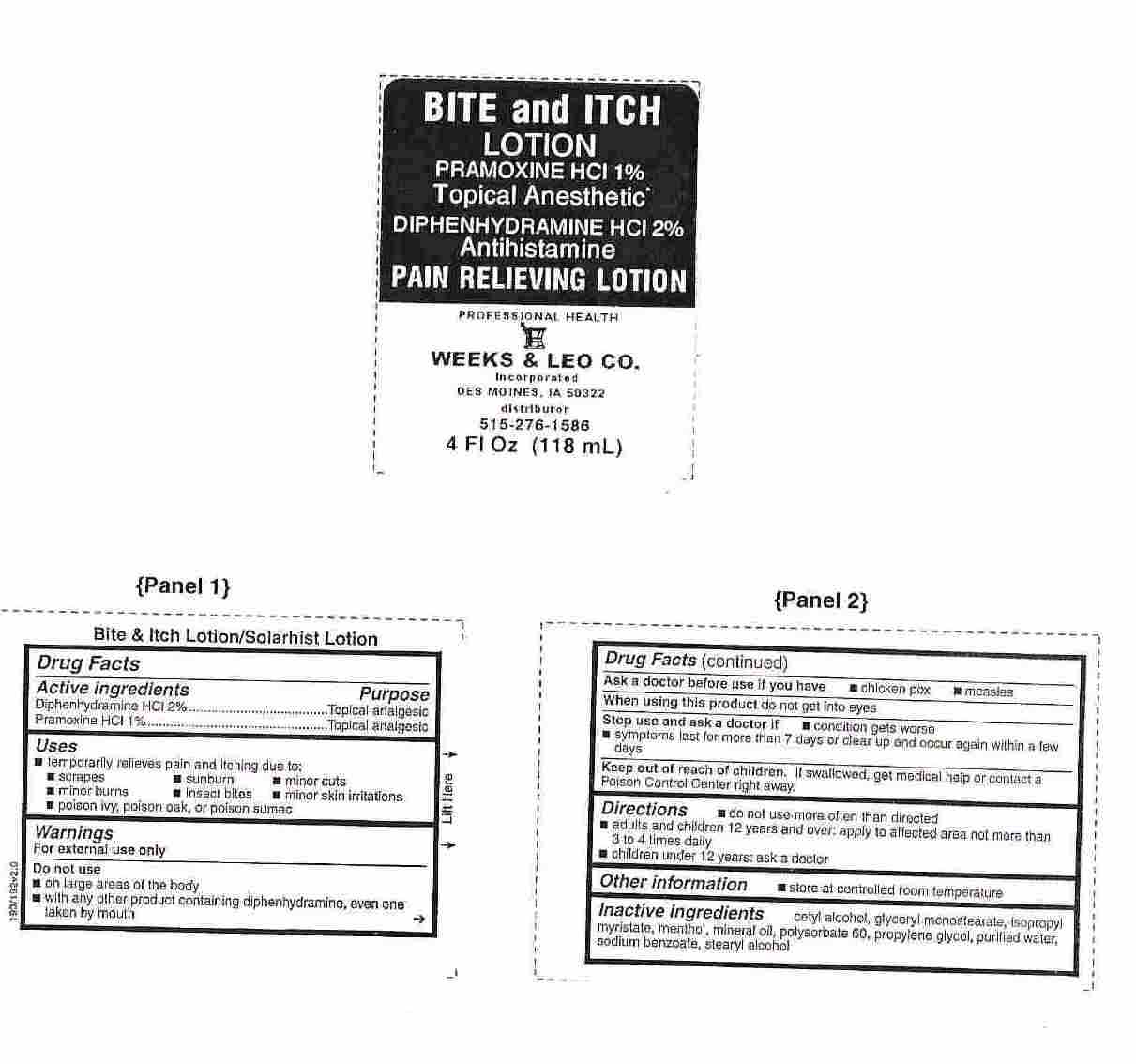
BITE AND ITCH- diphenhydramine hcl and pramoxine hcl lotion
Weeks & Leo Co., Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
temporarily relieves pain and itching due to:
- scrapes
- minor burns
- poison ivy, poison oak, or poison sumac
- sunburn
- insect bites
- minor cuts
- minor skin irritations
Warnings
For external use only
Do not use
- on large areas of the body
- with any other product containing diphenhydramine, even one taken by mouth
Directions
- do not use more often than directed
- adults and children 12 years and over: apply to affected areanot more than 3 to 4 times daily
- children under 12 years: ask a doctor
| BITE AND ITCH
diphenhydramine hcl and pramoxine hcl lotion |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Weeks & Leo Co., Inc. (005290028) |
| Registrant - DSC Laboratories, Div. of DSC Products Inc. (097807374) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DSC Laboratories, Div. of DSC Products Inc. | 097807374 | manufacture(11383-193) | |
Revised: 10/2016
Document Id: 3e4671ad-1de7-705e-e054-00144ff8d46c
Set id: 70c5bb8c-24d2-448b-a3b1-92c7f4754614
Version: 4
Effective Time: 20161007
Weeks & Leo Co., Inc.