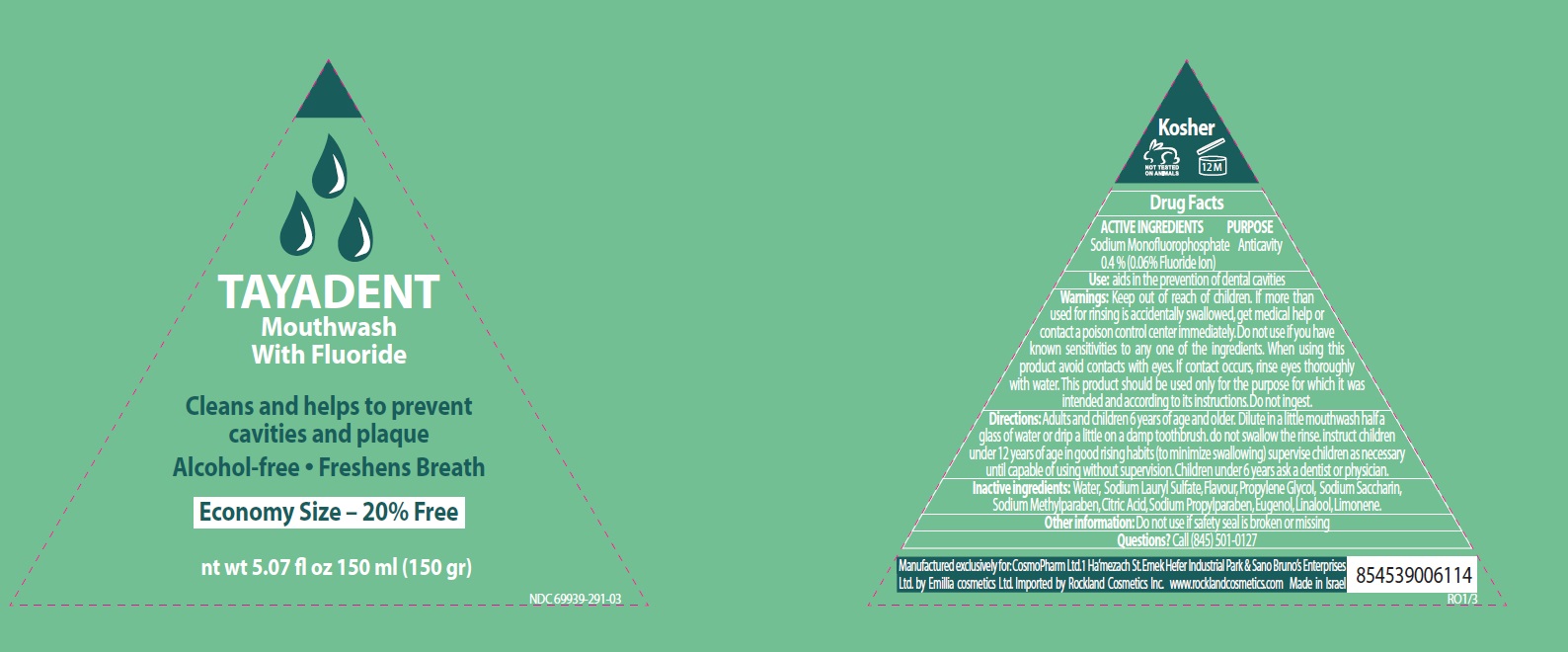
TAYADENT WITH FLUORIDE- sodium monofluorophosphate liquid
COSMOPHARM LTD.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Tayadent With Fluoride
Warnings
Directions
- Adults and children 6 years of age and older. Dilute in a little mouthwash half a glass of water or drip a little on a damp toothbrush. do not swallow the rinse
- instruct children under 12 years of age in good rising habits (to minimize swallowing) supervise children as necessary until capable of using without supervision
- Children under 6 years ask a dentist or physician
| TAYADENT WITH FLUORIDE
sodium monofluorophosphate liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - COSMOPHARM LTD. (600395487) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Emilia Cosmetics Ltd | 600076624 | manufacture(69939-291) | |
Revised: 8/2019
Document Id: 90b48bfc-2595-792d-e053-2995a90a2945
Set id: 70857412-8789-4045-baaf-62d1342a2ef9
Version: 3
Effective Time: 20190822
COSMOPHARM LTD.