Label: BENADRYL- diphenhydramine hydrochloride tablet, film coated
-
NDC Code(s):
50580-226-20,
50580-226-50,
50580-226-51,
50580-226-52, view more50580-226-53, 50580-226-54, 50580-226-56, 50580-226-62
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 29, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
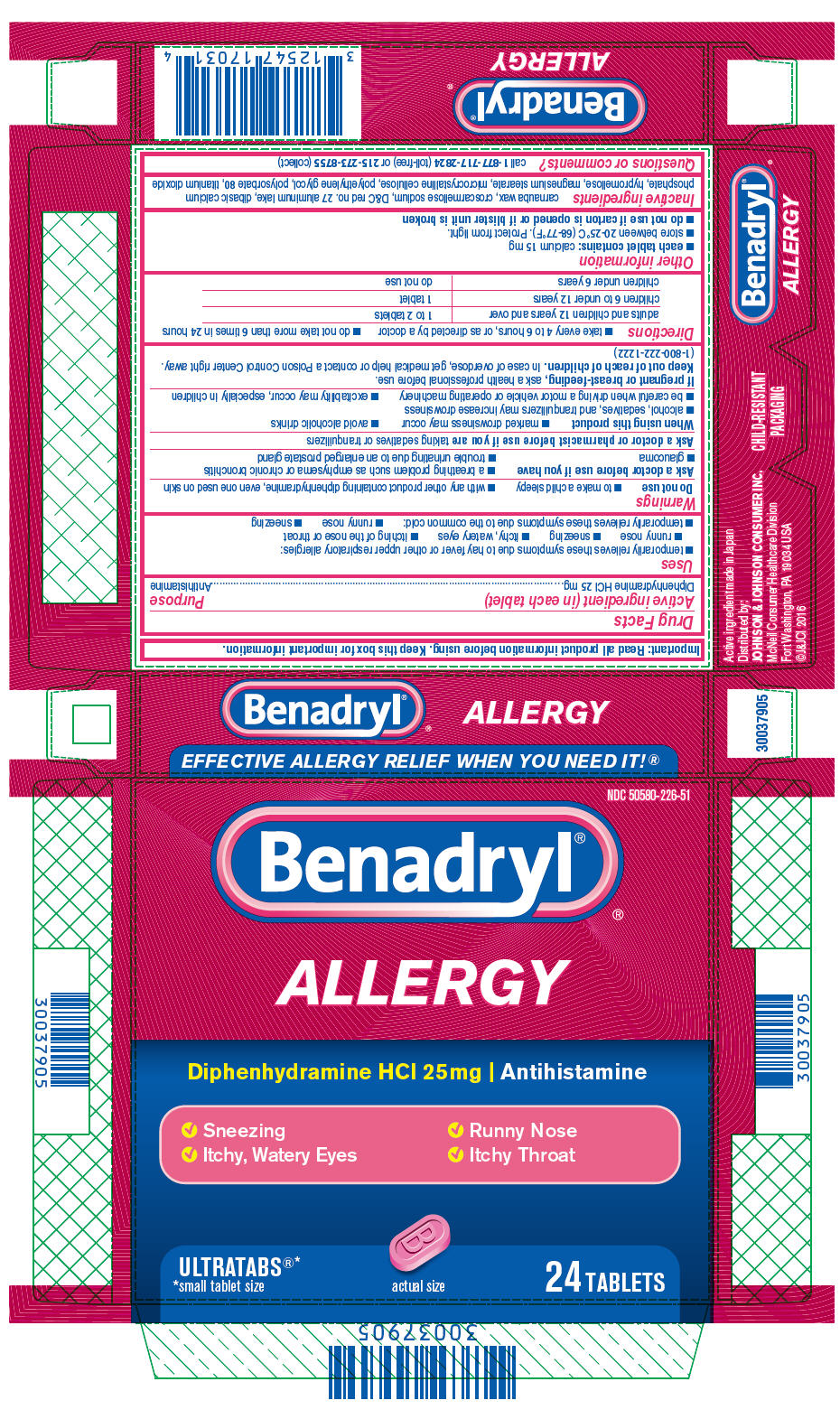
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BENADRYL
diphenhydramine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50580-226 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink Score no score Shape OVAL Size 11mm Flavor Imprint Code B;WL;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50580-226-50 1 in 1 CARTON 06/04/2012 1 100 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:50580-226-51 2 in 1 CARTON 06/04/2012 2 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:50580-226-53 2 in 1 POUCH; Type 0: Not a Combination Product 06/04/2012 4 NDC:50580-226-54 60 in 1 CARTON 07/27/2015 4 2 in 1 POUCH; Type 0: Not a Combination Product 5 NDC:50580-226-62 4 in 1 CARTON 01/02/2017 5 2 in 1 POUCH; Type 0: Not a Combination Product 6 NDC:50580-226-52 4 in 1 CARTON 06/04/2012 6 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 7 NDC:50580-226-56 3 in 1 PACKAGE 02/01/2013 7 4 in 1 CARTON 7 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 8 NDC:50580-226-20 2 in 1 CARTON 01/15/2024 8 1 in 1 CARTON 8 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 09/01/2008 Labeler - Johnson & Johnson Consumer Inc. (878046358)