BREATHRX WITH ZYTEX- sodium fluoride paste, dentifrice
Discus Dental, LLC
----------
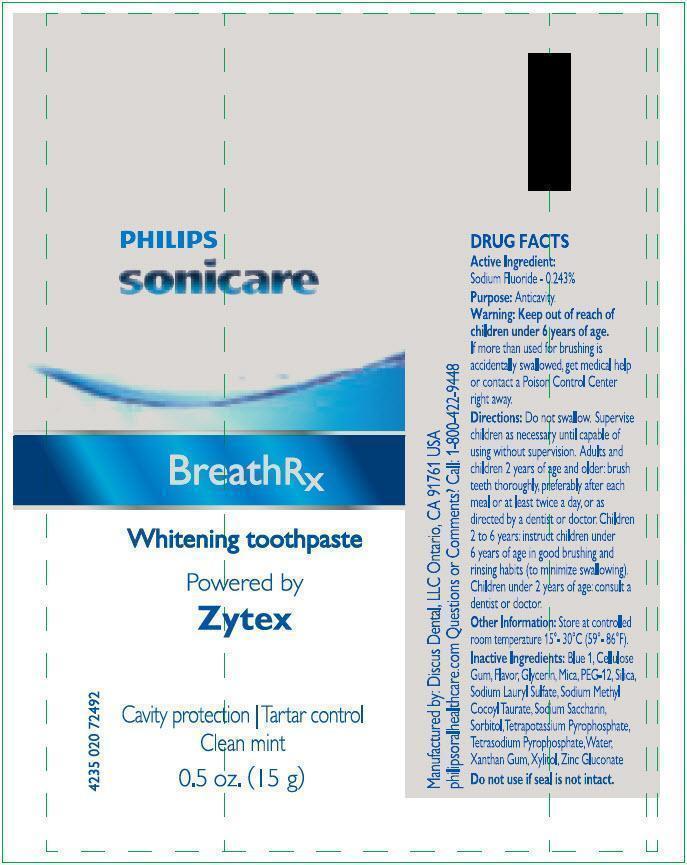
BreathRx with Zytex Whitening Toothpaste
Other Information
- Store at controlled room temperature 15 o - 30 oC (59 o - 86 oF)
Do not use if seal is not intact.
Inactive Ingredients
Blue 1,Cellulose Gum, Flavor (Mint, Thymol and Eucalyptus Oil), Glycerin, Mica & Titanium Dioxide, PEG-12, Silica, Sodium Lauryl Sulfate, Sodium Methyl Cocoyl Taurate, Sodium Saccharin, Sorbitol, Tetrapotassium Pyrophosphate, Tetrasodium Pyrophosphate, Water, Xanthan Gum, Xylitol, Zinc gluconate.
Warning: Keep out of reach of children under 6 years of age.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions:
- Do not swallow rinse.
- Supervise children as necessary until capable of using without supervision.
- Adults and children 2 years of age and older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
- Children 2 years to 6 years: Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing).
- Children under 2 years of age: Consult a dentist or doctor.
Twist off cap and remove foil seal. Do not use if seal is not intact.
| BREATHRX WITH ZYTEX
sodium fluoride paste, dentifrice |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Discus Dental, LLC (831726109) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Discus Dental, LLC | 831726109 | manufacture(64854-018) | |
Revised: 1/2024
Document Id: 0f162163-8d76-fe18-e063-6294a90a0127
Set id: 6fe909ef-b69c-4362-8724-4912b2197d72
Version: 7
Effective Time: 20240116
Discus Dental, LLC