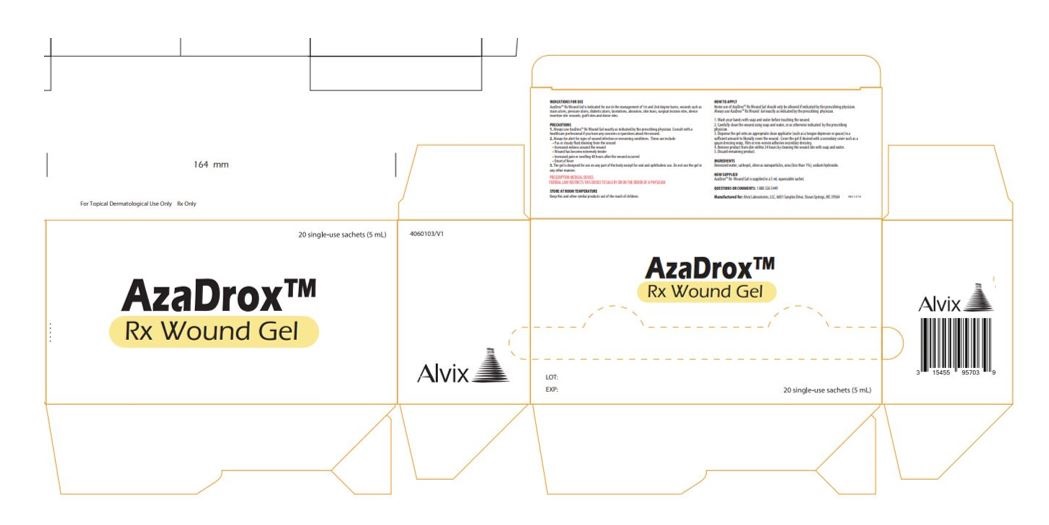
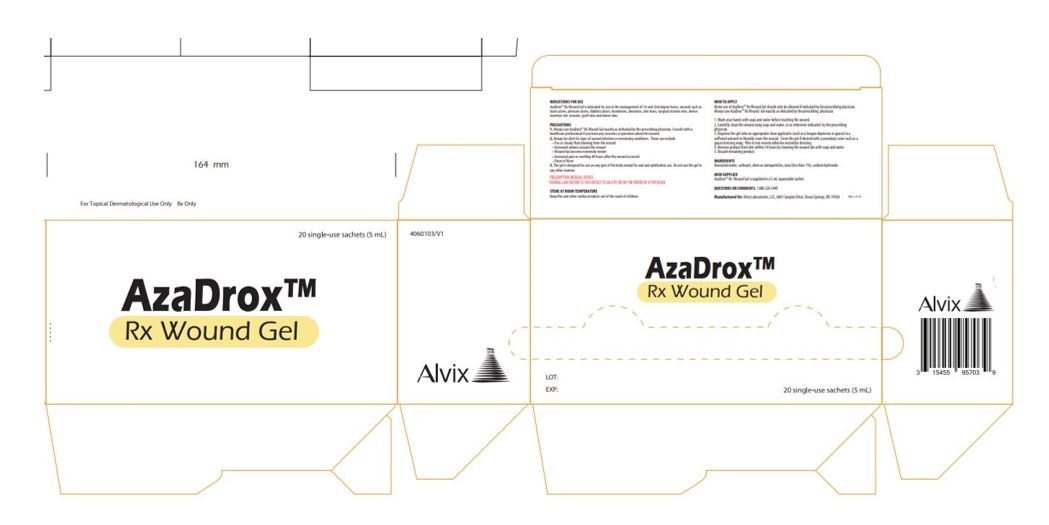
Label: AZADROX RX WOUND GEL-
- NDC Code(s): 00315455957015, 00315455957039
- Packager: Alvix Laboratories, LLC
- Category: PRESCRIPTION MEDICAL DEVICE LABEL
- DEA Schedule: None
- Marketing Status: Premarket Notification
Drug Label Information
Updated May 18, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
- INDICATIONS FOR USE
-
PRECAUTIONS
1. Always use AzaDrox™ Rx Wound Gel exactly as indicated by the prescribing physician. Consult with a healthcare professional if you have any concerns or questions about the wound.
2. Always be alert for signs of wound infection or worsening conditions. These can include:
• Pus or cloudy fluid draining from the wound
• Increased redness around the wound
• Wound has become extremely tender
• Increased pain or swelling 48 hours after wound occurred
• Onset of fever3. The gel is designed for use on any part of the body except for oral and opthalmic use. Do not use the gel in any other manner.
-
HOW TO APPLY
Home use of AzaDrox™ Rx Wound Gel should only be allowed if indicated by the prescribing physician. Always use AzaDrox™ Rx Wound Gel exactly as indicated by the prescribing physician.
1. Wash your hands with soap and water before touching the wound.2. Carefully clean the wound using soap and water, or as otherwise indicated by the prescribing physician.
3. Dispense gel onto appropriate clean applicator (such as a tongue depressor or gauze) in a sufficient amount to liberally cover the wound. Cover the gel if desired with a secondary cover such as a gauze dressing wrap, film, or non-woven adhesive secondary dressing.
4. Remove product from the skin within 24 hours by leaning the wound site with soap and water.
5. Discard remaining product.
- INGREDIENTS
- HOW SUPPLIED
- PRESCRIPTION MEDICAL DEVICE: FEDERAL LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN
- AzaDrox™ Rx Wound Gel Package Insert
- AzaDrox™ Rx Wound Gel Primary Label
-
INGREDIENTS AND APPEARANCE
AZADROX RX WOUND GEL
dressing, wound, drugProduct Information Product Type PRESCRIPTION MEDICAL DEVICE Item Code (Source) GS1:00315455957039 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 GS1:00315455957015 5 in 1 PACKET 1 GS1:00315455957039 100 mL in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K162017 05/18/2020 Labeler - Alvix Laboratories, LLC (962445925)