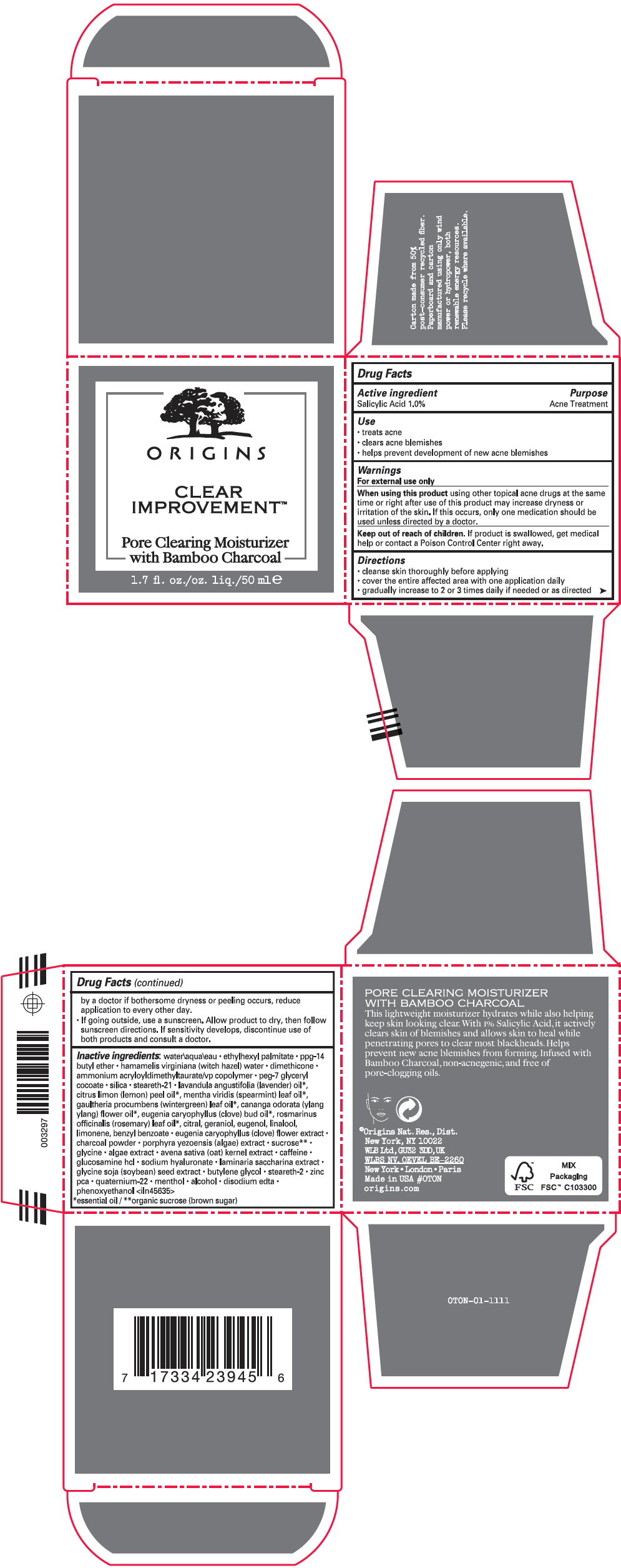
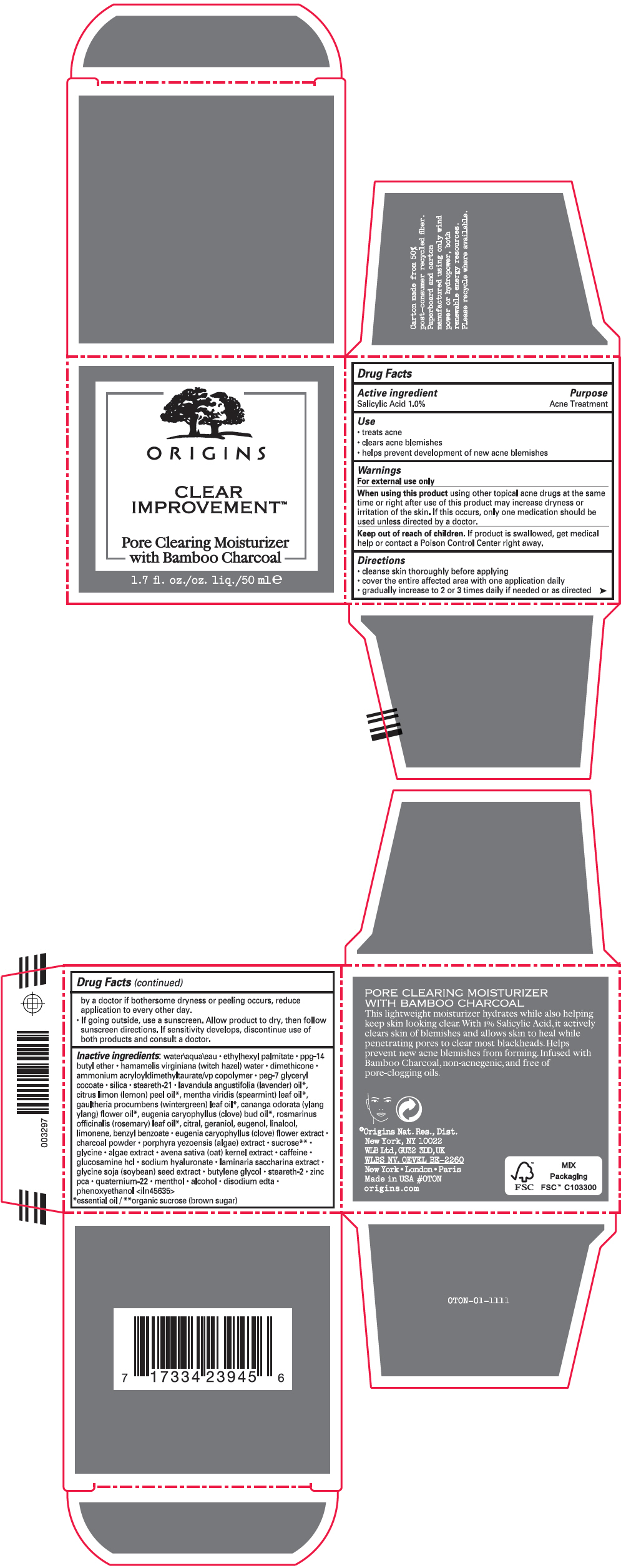
Label: CLEAR IMPROVEMENT PORE CLEARING MOISTURIZER- salicylic acid lotion
- NDC Code(s): 59427-015-01, 59427-015-02
- Packager: ORIGINS NATURAL RESOURCES INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
- cleanse skin thoroughly before applying
- cover the entire affected area with one application daily
- gradually increase to 2 or 3 times daily if needed or as directed by a doctor if bothersome dryness or peeling occurs, reduce application to every other day.
- If going outside, use a sunscreen. Allow product to dry, then follow sunscreen directions. If sensitivity develops, discontinue use of both products and consult a doctor.
-
Inactive ingredients
water\aqua\eau • ethylhexyl palmitate • ppg-14 butyl ether • hamamelis virginiana (witch hazel) water • dimethicone • ammonium acryloyldimethyltaurate/vp copolymer • peg-7 glyceryl cocoate • silica • steareth-21 • lavandula angustifolia (lavender) oil 1, citrus limon (lemon) peel oil 1, mentha viridis (spearmint) leaf oil 1, gaultheria procumbens (wintergreen) leaf oil 1, cananga odorata (ylang ylang) flower oil 1, eugenia caryophyllus (clove) bud oil 1, rosmarinus officinalis (rosemary) leaf oil 1, citral, geraniol, eugenol, linalool, limonene, benzyl benzoate • eugenia caryophyllus (clove) flower extract • charcoal powder • porphyra yezoensis (algae) extract • sucrose 2 • glycine • algae extract • avena sativa (oat) kernel extract • caffeine • glucosamine hcl • sodium hyaluronate • laminaria saccharina extract • glycine soja (soybean) seed extract • butylene glycol • steareth-2 • zinc pca • quaternium-22 • menthol • alcohol • disodium edta • phenoxyethanol <iln45635>
- PRINCIPAL DISPLAY PANEL - 50 ml Jar Carton
-
INGREDIENTS AND APPEARANCE
CLEAR IMPROVEMENT PORE CLEARING MOISTURIZER
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59427-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-21 (UNII: 53J3F32P58) LAVENDER OIL (UNII: ZBP1YXW0H8) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) SPEARMINT OIL (UNII: C3M81465G5) METHYL SALICYLATE (UNII: LAV5U5022Y) YLANG-YLANG OIL (UNII: 8YOY78GNNX) CLOVE OIL (UNII: 578389D6D0) ROSEMARY OIL (UNII: 8LGU7VM393) CITRAL (UNII: T7EU0O9VPP) GERANIOL (UNII: L837108USY) EUGENOL (UNII: 3T8H1794QW) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) BENZYL BENZOATE (UNII: N863NB338G) CLOVE (UNII: K48IKT5321) ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) PHYMATOLITHON CALCAREUM (UNII: 6J1M3WA0ZK) SUCROSE (UNII: C151H8M554) GLYCINE (UNII: TE7660XO1C) OAT (UNII: Z6J799EAJK) CAFFEINE (UNII: 3G6A5W338E) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) SOYBEAN (UNII: L7HT8F1ZOD) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) STEARETH-2 (UNII: V56DFE46J5) ZINC PIDOLATE (UNII: C32PQ86DH4) QUATERNIUM-22 (UNII: MXO138JCBP) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) ALCOHOL (UNII: 3K9958V90M) EDETATE DISODIUM (UNII: 7FLD91C86K) PHENOXYETHANOL (UNII: HIE492ZZ3T) WITCH HAZEL (UNII: 101I4J0U34) WATER (UNII: 059QF0KO0R) ETHYLHEXYL PALMITATE (UNII: 2865993309) PPG-14 BUTYL ETHER (UNII: R199TJT95T) DIMETHICONE (UNII: 92RU3N3Y1O) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) PEG-7 GLYCERYL COCOATE (UNII: VNX7251543) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59427-015-01 1 in 1 CARTON 09/23/2019 1 50 mL in 1 JAR; Type 0: Not a Combination Product 2 NDC:59427-015-02 15 mL in 1 TUBE; Type 0: Not a Combination Product 12/08/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/23/2019 Labeler - ORIGINS NATURAL RESOURCES INC. (611716283) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(59427-015) , label(59427-015) , pack(59427-015)