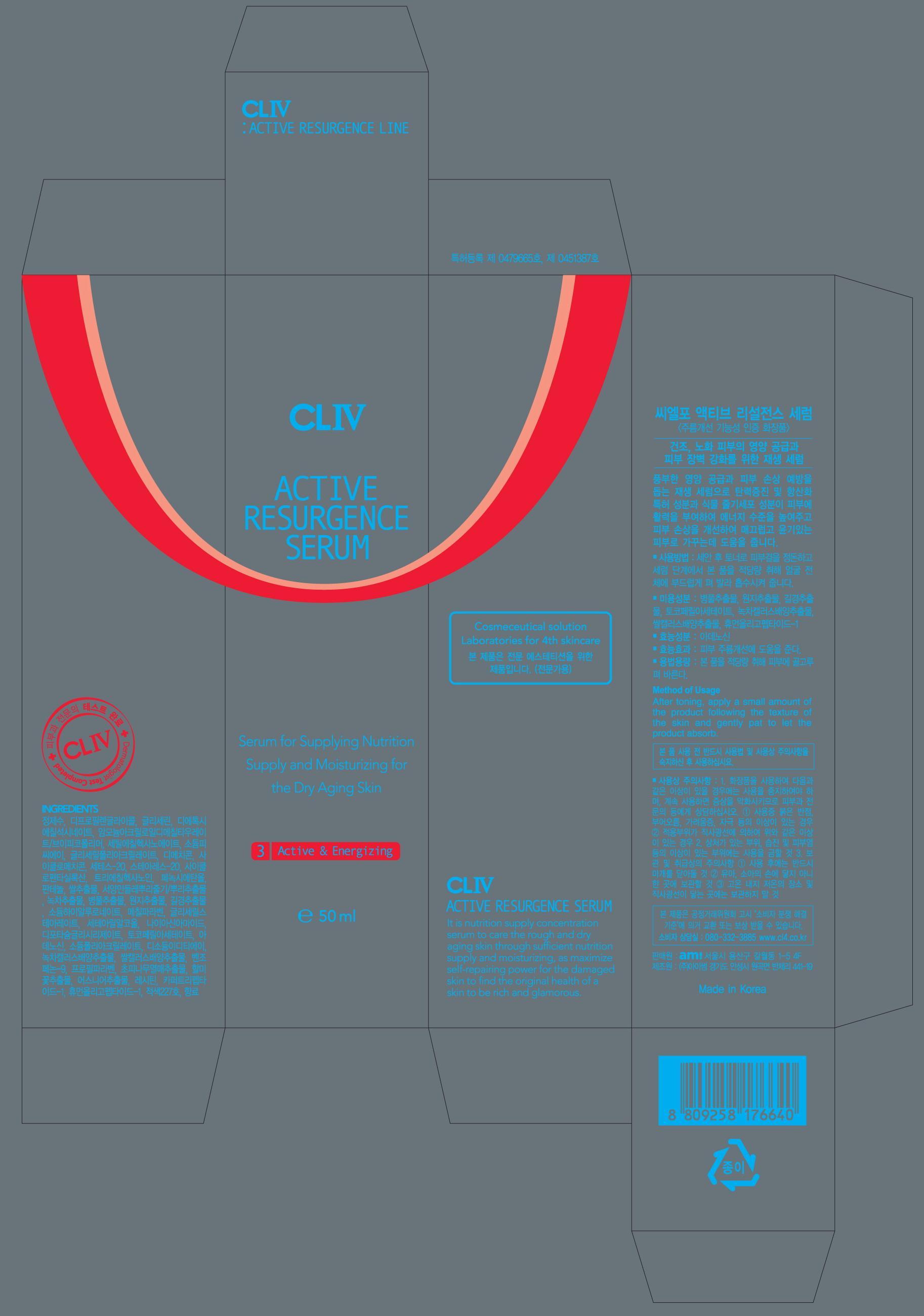
ACTIVE RESURGENCE SERUM- glycerin liquid
AMI Cosmetic Co.,Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
water, dipropylene glycol, glycerin, diethoxyethyl succinate, ammonium acryloyldimethyltaurate/vp copolymer, cetyl ethylhexanoate, sodium pca, glyceryl polyacrylate, dimethicone, cyclomethicone, ceteth-20, steareth-20 cyclopentasiloxane, triethylhexanion, panthenol, rice extract, dandelion rhizome/root extract, camellia sinensis leaf extract, centella asiatica extract, polygala tenuifolia root extract, platycodon grandiflorum root extract, sodium hyaluronate, glyceryl stearate, cetearyl alcohol, niacinamide, dipotassium glycyrrhizate, tocopheryl acetate, adenosine, sodium polyacrylate, disodium edta, camellia sinensis callus culture extract, rice callus culture extract, benzophenone-9, zanthoxylum piperitum fruit extract, pulsatilla koreana extract, lichen extract, lecithin, copper tripeptide-1, phenoxyethanol, methylparaben, propylparaben, red 33, fragrance
supplying nutrition and moisturizing for the dry aging skin
keep out of reach of the children
after toning, apply a small amount of the product following the texture of the skin and gently pat to let the product absorb
・Stop using the product when you have skin
problems or the product disagrees with your skin.
・Stop using the product immediately and consult a
dermatologist if you have redness, swelling,
itching or irritation on the skin while or after
using the product.
・If the product gets into the eyes, don't rub but
rinse with water.
・Don't place the product in any place where it will
be subjected to extremely high or low
temperatures or direct sunlight.
AMI Cosmetic Co.,Ltd.