SENNA- sennosides tablet
STRATEGIC SOURCING SERVICES LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
healthmart 451
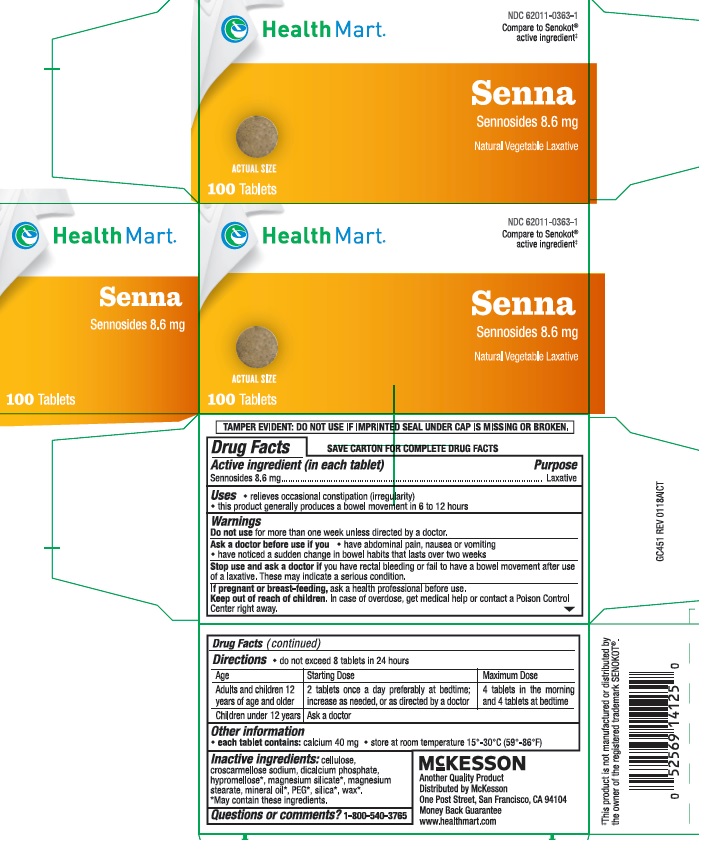
Uses
- this product generally produces a bowel movement in 6 to 12 hours
- relieves occasional constipation (irregularity)
Warnings
Do not use for more than one week unless directed by a doctor.
Ask a doctor before use if you
- have abdominal pain, nausea or vomiting
- have noticed a sudden change in bowel habits that lasts over two weeks
Stop use and ask a doctor if you have rectal bleeding or fail to have a bowel movement after use if a laxative. These may indicate a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Directions
- do not exceed 8 tablets in 24 hours
| Age | Starting Dose | Maximum Dose |
|---|---|---|
| adults and children 12 years of age and older | 2 tablets once a day preferably at bedtime; increase as needed, or as directed by a doctor | 4 tablets in the morning and 4 tablets at bedtime |
| children under 12 years | ask a doctor |
| SENNA
sennosides tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - STRATEGIC SOURCING SERVICES LLC (116956644) |
| Registrant - Geri-Care Pharmaceutical Corp (611196254) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Geri-Care Pharmaceuticals, Corp | 611196254 | repack(62011-0363) | |
Revised: 12/2019
Document Id: 9aa0a10a-50df-4d29-e053-2995a90aa32d
Set id: 6e9c5e94-9b99-706a-e053-2991aa0a052d
Version: 2
Effective Time: 20191226
STRATEGIC SOURCING SERVICES LLC