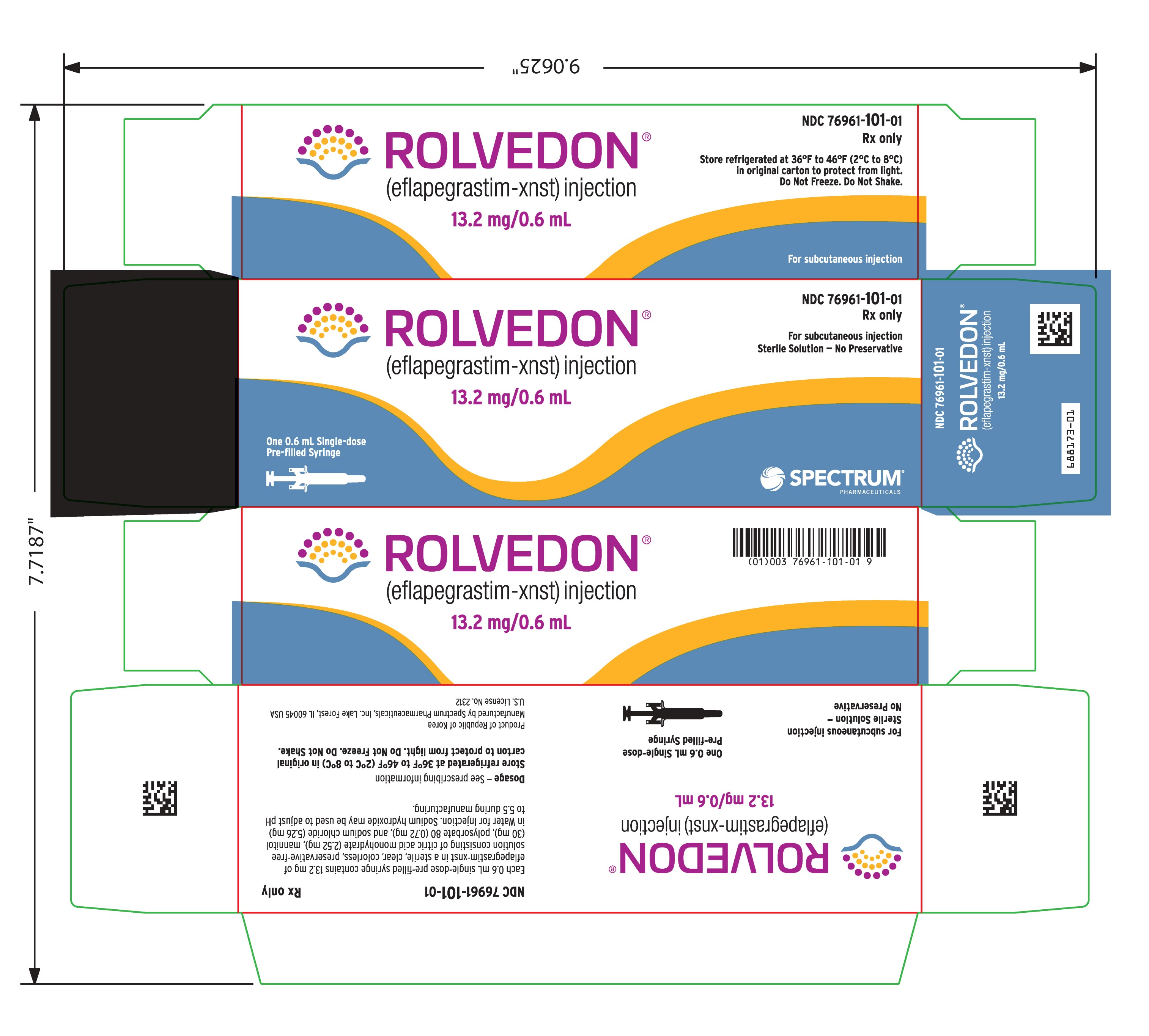
Label: ROLVEDON- eflapegrastim-xnst injection, solution
- NDC Code(s): 76961-101-01
- Packager: Spectrum Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 8, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ROLVEDON® safely and effectively. See full prescribing information for ROLVEDON.
ROLVEDON® (eflapegrastim-xnst) injection, for subcutaneous use
Initial U.S. Approval: 2022RECENT MAJOR CHANGES
Dosage and Administration, Administration (2.2) 06/2023
INDICATIONS AND USAGE
Rolvedon is a leukocyte growth factor indicated to decrease the incidence of infection, as manifested by febrile neutropenia, in adult patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with clinically significant incidence of febrile neutropenia. (1)
Limitations of Use
Rolvedon is not indicated for the mobilization of peripheral blood progenitor cells for hematopoietic stem cell transplantation. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 13.2 mg/0.6 mL solution in a single-dose prefilled syringe. (3)
CONTRAINDICATIONS
Patients with a history of serious allergic reactions to human granulocyte colony-stimulating factors such as eflapegrastim, pegfilgrastim or filgrastim products. (4)
WARNINGS AND PRECAUTIONS
- •
- Fatal splenic rupture: Evaluate patients who report left upper abdominal or shoulder pain for an enlarged spleen or splenic rupture. (5.1)
- •
- Acute respiratory distress syndrome (ARDS): Evaluate patients who develop fever, lung infiltrates, or respiratory distress. Discontinue Rolvedon in patients with ARDS. (5.2)
- •
- Serious allergic reactions, including anaphylaxis: Permanently discontinue Rolvedon in patients with serious allergic reactions. (5.3)
- •
- Sickle Cell Crisis in Patients with Sickle Cell Disorders: Discontinue Rolvedon if sickle cell crisis occurs. (5.4)
- •
- Glomerulonephritis: Evaluate and consider dose-reduction or interruption of Rolvedon if causality is likely. (5.5)
- •
- Leukocytosis: Monitor complete blood count (CBC) during Rolvedon therapy. (5.6)
- •
- Thrombocytopenia: Monitor platelet counts. (5.7)
- •
- Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML): Monitor patients with breast and lung cancer using Rolvedon in conjunction with chemotherapy and/or radiotherapy for signs and symptoms of MDS/AML. (5.10)
ADVERSE REACTIONS
The most common adverse reactions (≥20%) are fatigue, nausea, diarrhea, bone pain, headache, pyrexia, anemia, rash, myalgia, arthralgia, and back pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Spectrum Pharmaceuticals, Inc. at 1-888-713-0688 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Splenic Rupture
5.2 Acute Respiratory Distress Syndrome
5.3 Serious Allergic Reactions
5.4 Sickle Cell Crisis in Patients with Sickle Cell Disorders
5.5 Glomerulonephritis
5.6 Leukocytosis
5.7 Thrombocytopenia
5.8 Capillary Leak Syndrome
5.9 Potential for Tumor Growth Stimulatory Effects on Malignant Cells
5.10 Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML) in Patients with Breast and Lung Cancer
5.11 Aortitis
5.12 Nuclear Imaging
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Rolvedon is indicated to decrease the incidence of infection, as manifested by febrile neutropenia, in adult patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with clinically significant incidence of febrile neutropenia.
Limitations of Use
Rolvedon is not indicated for the mobilization of peripheral blood progenitor cells for hematopoietic stem cell transplantation.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of Rolvedon is a single subcutaneous injection of 13.2 mg administered once per chemotherapy cycle. Administer approximately 24 hours after cytotoxic chemotherapy. Do not administer within the period from 14 days before to 24 hours after administration of cytotoxic chemotherapy.
2.2 Administration
Rolvedon is administered subcutaneously via a single-dose prefilled syringe.
Prior to use‚ take the carton out of the refrigerator and place the sealed blister tray on a clean flat surface for a minimum of 30 minutes to allow the product to reach room temperature. Do not warm up the prefilled syringe in any other way. Discard any prefilled syringe left at room temperature for greater than 12 hours. Do not shake. If Rolvedon is accidentally frozen, do not use. Remove the tray from box and carefully remove the prefilled syringe from the tray. If you drop the prefilled syringe onto a hard surface, do not use it. Use a new syringe for the injection.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not administer Rolvedon if discoloration or particulates are observed.
Administer the entire contents of the prefilled syringe.
If the patient or caregiver misses a dose of Rolvedon, instruct them to contact their healthcare provider.
The Rolvedon prefilled syringe does not bear graduation marks and is intended only to deliver the entire contents of the syringe (13.2 mg/0.6 mL) for direct administration.
Not made with natural rubber latex.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Rolvedon is contraindicated in patients with a history of serious allergic reactions to eflapegrastim, pegfilgrastim, or filgrastim products. Reactions may include anaphylaxis [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Splenic Rupture
Splenic rupture, including fatal cases, can occur following the administration of recombinant human granulocyte colony-stimulating factor (rhG-CSF) products, such as Rolvedon. Evaluate for an enlarged spleen or splenic rupture in patients who report left upper abdominal or shoulder pain after receiving Rolvedon.
5.2 Acute Respiratory Distress Syndrome
Acute respiratory distress syndrome (ARDS) can occur in patients receiving rhG-CSF products, such as Rolvedon. Evaluate patients who develop fever and lung infiltrates or respiratory distress after receiving Rolvedon for ARDS. Discontinue Rolvedon in patients with ARDS.
5.3 Serious Allergic Reactions
Serious allergic reactions, including anaphylaxis, can occur in patients receiving rhG-CSF products, such as Rolvedon. Permanently discontinue Rolvedon in patients with serious allergic reactions. Rolvedon is contraindicated in patients with a history of serious allergic reactions to eflapegrastim, pegfilgrastim, or filgrastim products [see Contraindications (4)].
5.4 Sickle Cell Crisis in Patients with Sickle Cell Disorders
Severe and sometimes fatal sickle cell crises can occur in patients with sickle cell disorders receiving rhG-CSF products, such as Rolvedon. Discontinue Rolvedon if sickle cell crisis occurs.
5.5 Glomerulonephritis
Glomerulonephritis has occurred in patients receiving rhG-CSF products. The diagnoses were based upon azotemia, hematuria (microscopic and macroscopic), proteinuria, and renal biopsy. Generally, events of glomerulonephritis resolved after dose-reduction or discontinuation of rhG-CSF. If glomerulonephritis is suspected, evaluate for cause. If causality is likely, consider dose-reduction or interruption of Rolvedon.
5.6 Leukocytosis
White blood cell (WBC) counts of 100 x 109/L or greater have been observed in patients receiving rhG-CSF products. Monitor complete blood count (CBC) during Rolvedon therapy. Discontinue Rolvedon treatment if WBC count of 100 x 109/L or greater occurs.
5.7 Thrombocytopenia
Thrombocytopenia has been reported in patients receiving rhG-CSF products. Monitor platelet counts.
5.8 Capillary Leak Syndrome
Capillary leak syndrome has been reported after administration of rhG-CSF products and is characterized by hypotension, hypoalbuminemia, edema and hemoconcentration. Episodes vary in frequency and severity, and may be life-threatening if treatment is delayed. Patients who develop symptoms of capillary leak syndrome should be closely monitored and receive standard symptomatic treatment, which may include a need for intensive care.
5.9 Potential for Tumor Growth Stimulatory Effects on Malignant Cells
The granulocyte colony-stimulating factor (G-CSF) receptor through which Rolvedon acts has been found on tumor cell lines. The possibility that Rolvedon acts as a growth factor for any tumor type, including myeloid malignancies and myelodysplasia, diseases for which Rolvedon is not approved, cannot be excluded.
5.10 Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML) in Patients with Breast and Lung Cancer
MDS and AML have been associated with the use of rhG-CSF products in conjunction with chemotherapy and/or radiotherapy in patients with breast and lung cancer. Monitor patients for signs and symptoms of MDS/AML in these settings.
5.11 Aortitis
Aortitis has been reported in patients receiving rhG-CSF products. It may occur as early as the first week after start of therapy. Manifestations may include generalized signs and symptoms such as fever, abdominal pain, malaise, back pain, and increased inflammatory markers (e.g., c-reactive protein and white blood cell count). Consider aortitis in patients who develop these signs and symptoms without known etiology. Discontinue Rolvedon if aortitis is suspected.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- •
- Splenic rupture [see Warnings and Precautions (5.1)]
- •
- Acute respiratory distress syndrome [see Warnings and Precautions (5.2)]
- •
- Serious allergic reactions [see Warnings and Precautions (5.3)]
- •
- Sickle cell crisis in patients with sickle cell disorders [see Warnings and Precautions (5.4)]
- •
- Glomerulonephritis [see Warnings and Precautions (5.5)]
- •
- Leukocytosis [see Warnings and Precautions (5.6)]
- •
- Thrombocytopenia [see Warnings and Precautions (5.7)]
- •
- Capillary leak syndrome [see Warnings and Precautions (5.8)]
- •
- Potential for tumor growth stimulatory effects on malignant cells [see Warnings and Precautions (5.9)]
- •
- Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML) in Patients with Breast and Lung Cancer [see Warnings and Precautions (5.10)]
- •
- Aortitis [see Warnings and Precautions (5.11)]
- •
- Nuclear Imaging [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of Rolvedon was evaluated in Study 1 and Study 2 [see Clinical Studies (14)]. Patients with early-stage breast cancer received Rolvedon 13.2 mg by subcutaneous injection (n=314) or pegfilgrastim 6 mg by subcutaneous injection (n=326) on Day 2 of each cycle after docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 (TC) chemotherapy.
Among patients receiving Rolvedon, a total of 272 patients received four 21-day treatment cycles. The most common adverse reactions (≥20%) were fatigue, nausea, diarrhea, bone pain, headache, pyrexia, anemia, rash, myalgia, arthralgia, and back pain. Table 1 summarizes the adverse reactions that occurred in Studies 1 and 2.
Table 1. Common Adverse Reactions with a Frequency of ≥10% Through Week 14 in Patients with Early-Stage Breast Cancer in Study 1 and Study 2 Adverse Reaction Rolvedon
(N = 314)
%Pegfilgrastim**
(N=326)
%*Grouped Terms **Study 1 and Study 2 were not designed to evaluate meaningful comparisons of the incidence of adverse reactions in the Rolvedon and the pegfilgrastim treatment groups. Fatigue *
181 (58%)
192 (59%)
Nausea
162 (52%)
166 (51%)
Diarrhea
125 (40%)
126 (39%)
Bone pain
119 (38%)
121 (37%)
Headache*
92 (29%)
90 (28%)
Pyrexia *
87 (28%)
84 (26%)
Anemia*
77 (25%)
52 (16%)
Rash*
77 (25%)
99 (30%)
Myalgia
69 (22%)
49 (15%)
Arthralgia
66 (21%)
48 (15%)
Back pain*
63 (20%)
55 (17%)
Decreased appetite
61 (19%)
50 (15%)
Peripheral edema*
57 (18%)
53 (16%)
Abdominal pain*
53 (17%)
67 (21%)
Dizziness *
50 (16%)
38 (12%)
Dyspnea*
49 (16%)
44 (13%)
Cough*
48 (15%)
51 (16%)
Thrombocytopenia*
44 (14%)
17 (5%)
Pain
37 (12%)
42 (13%)
Pain in extremity
36 (11%)
42 (13%)
Local administration reactions*
34 (11%)
27 (8%)
Flushing
32 (10%)
27 (8%)
Permanent discontinuation due to an adverse reaction occurred in 4% of patients who received Rolvedon. The adverse reaction requiring permanent discontinuation in 3 patients who received Rolvedon was rash.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on Rolvedon use in pregnant women; however, data from published studies with use of other recombinant human granulocyte colony-stimulating factor (rhG-CSF) products in pregnant women have not identified any drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes.
Animal reproduction studies were conducted in rats and rabbits. In rats, eflapegrastim-xnst did not adversely affect embryofetal and/or postnatal development when administered from organogenesis throughout lactation at doses that produced maternal exposures up to 7 times the exposure at the recommended clinical dose. In rabbits, eflapegrastim-xnst caused embryofetal lethality and reduced fetal weight when administered during the organogenesis period at approximately 6 times the exposure at the clinical dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In an embryofetal developmental study in rabbits, eflapegrastim-xnst was administered subcutaneously every other day during the period of organogenesis at doses up to 10 times the clinical exposure at the maximum recommended dose of 13.2 mg. Increased post-implantation loss, reduced number of live fetuses, and reduced fetal body weights were observed at 6 times the clinical exposure, based on AUC. No malformations were observed up to 10 times the clinical exposure, based on AUC.
In an embryofetal developmental study in rats, eflapegrastim-xnst administered subcutaneously every other day during the period of organogenesis did not adversely affect embryofetal development at doses up to 7 times clinical exposure, based on AUC.
In a pre- and post-natal development study in rats, eflapegrastim-xnst administered subcutaneously once weekly from organogenesis through lactation did not adversely affect behavioral, developmental, or reproductive parameters at doses up to 7 times the clinical exposure, based on AUC.
8.2 Lactation
Risk Summary
There are no data on the presence of eflapegrastim-xnst in human milk, the effects on the breastfed child, or the effects on milk production. Endogenous granulocyte colony-stimulating factor (G-CSF) is present in human milk. Other recombinant human granulocyte colony-stimulating factor (rhG-CSF) products are present in human milk at low levels and are not orally absorbed by infants. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Rolvedon and any potential adverse effects on the breastfed child from Rolvedon or from the underlying maternal condition.
-
10 OVERDOSAGE
Overdose of Rolvedon may result in leukocytosis and bone pain. In the event of overdose, general supportive measures should be instituted as necessary. Monitor the patient for adverse reactions [see Adverse Reactions (6)].
-
11 DESCRIPTION
Eflapegrastim-xnst is a granulocyte colony-stimulating factor (G‑CSF) produced by covalent coupling of a human G-CSF analog (18.6 kDa) and an Fc fragment of human immunoglobulin G4 (IgG4) (49.8 kDa), both derived from recombinant E. coli, via a single 3.4 kDa polyethylene glycol linker. The recombinant G-CSF domain in eflapegrastim-xnst is a variant of human G-CSF with two serine substitutions at positions 17 and 65, and no additional N-terminal methionine. Eflapegrastim-xnst has a molecular weight of approximately 72 kDa.
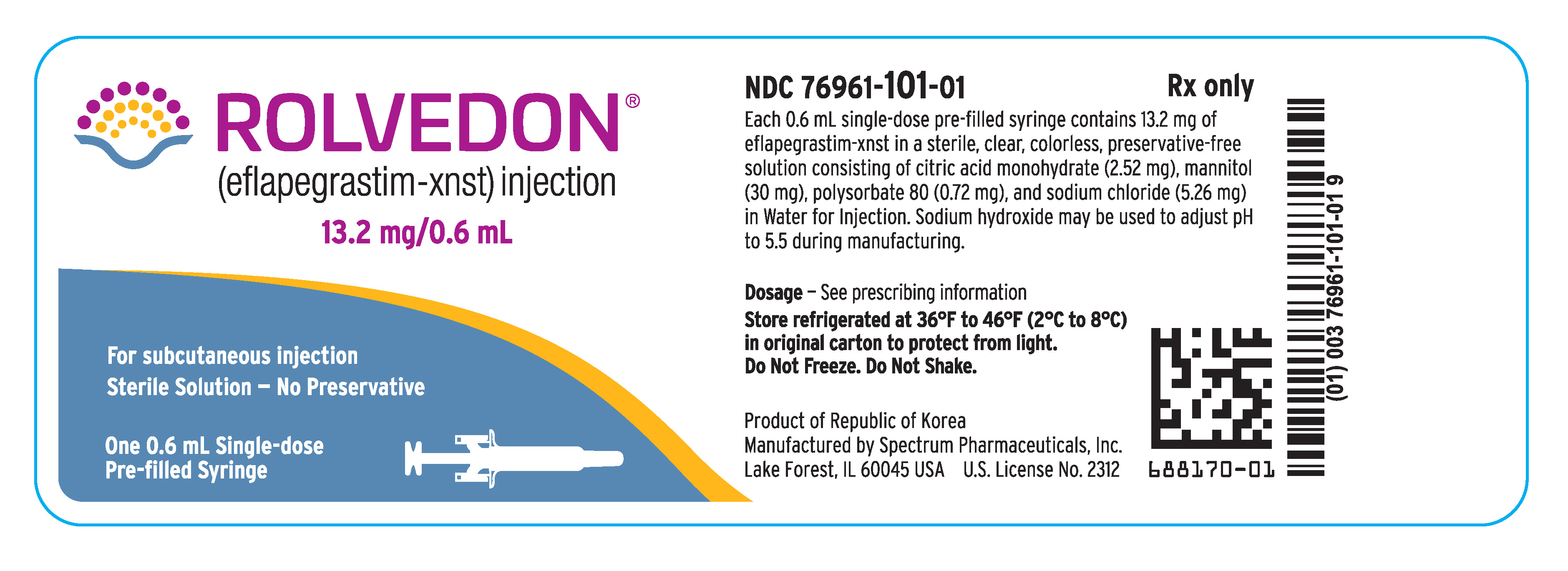
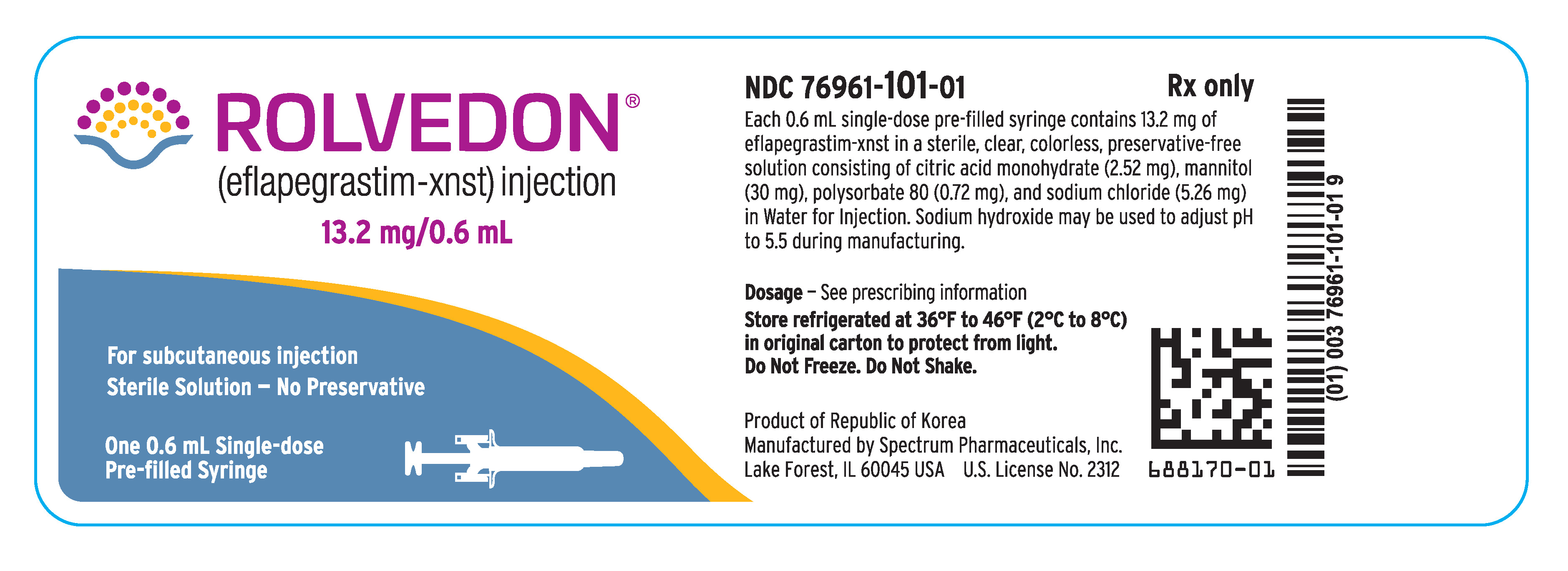
Rolvedon (eflapegrastim-xnst) injection is a sterile, preservative-free, clear, colorless solution supplied in a single-dose prefilled syringe for subcutaneous use. Each 0.6 mL single-dose prefilled syringe contains 13.2 mg of eflapegrastim-xnst, citric acid monohydrate (2.52 mg), mannitol (30 mg), polysorbate 80 (0.72 mg) and sodium chloride (5.26 mg) in Water for Injection. Sodium hydroxide may be used to adjust pH to 5.5 during manufacturing.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Eflapegrastim-xnst is a recombinant human granulocyte growth factor that binds to G-CSF receptors on myeloid progenitor cells and neutrophils, triggering signaling pathways that control cell differentiation, proliferation, migration and survival.
12.2 Pharmacodynamics
Eflapegrastim-xnst has been shown to elevate neutrophil counts in healthy subjects and in cancer patients. Absolute neutrophil count (ANC), Cmax and area under the effect curve (AUEClast) increased with increasing doses of eflapegrastim-xnst in a linear, but less than dose-proportional, manner over a dose range of 45 to 350 mcg/kg.
12.3 Pharmacokinetics
The pharmacokinetics of eflapegrastim-xnst was studied in healthy subjects and patients with breast cancer. After subcutaneous (SC) dosing, the pharmacokinetics of eflapegrastim-xnst was nonlinear and exposure increases were not dose-proportional over the dose range of 45 to 350 mcg/kg.
Absorption
The median Tmax of eflapegrastim-xnst is 25 hours (6 to 144 hours) in patients with breast cancer following administration of the recommended dosage.
Distribution
The volume of distribution of eflapegrastim-xnst is 1.44 L.
Elimination
The geometric mean half-life of eflapegrastim-xnst in patients with breast cancer is 36.4 hours (Range: 16.1 to 115 hours) during Cycle 1. Eflapegrastim-xnst clearance decreased with increasing doses following single dose administration, suggesting target-mediated clearance of eflapegrastim-xnst by neutrophils. Following repeat administration, clearance increased in Cycle 3 as compared to Cycle 1, potentially due to the subsequent increase in neutrophils. In in vitro studies, the IgG4 Fc fragment in eflapegrastim-xnst binds to the neonatal Fc receptor (FcRn), facilitating the FcRn-mediated transcytosis of eflapegrastim-xnst.
Metabolism
Eflapegrastim-xnst is expected to be metabolized by endogenous degradation following receptor-mediated internalization by cells bearing the G-CSF receptor.
Excretion
Eflapegrastim-xnst was not detected in urine.
Drug Interaction Studies
No studies evaluating the drug interaction potential of eflapegrastim-xnst have been conducted.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of eflapegrastim-xnst or of other eflapegrastim products.
Antibodies to eflapegrastim-xnst were detected using bridging enzyme-linked immunosorbent assay (ELISA) with a sensitivity of 65 ng/mL. During the 12-week treatment period in the two randomized studies, twenty-one of 297 (7.1%) patients treated with eflapegrastim-xnst developed antibodies. At the 12-month follow-up visit after the last dose of treatment in the two randomized studies, 28 of 297 (9.4%) patients treated with eflapegrastim-xnst developed antibodies.
Neutralizing antibodies were detected by a cell-based assay with a sensitivity of 1.95 mcg/mL. One patient out of 297 (0.3%) in the eflapegrastim-xnst arm tested positive for neutralizing antibodies post-treatment. Treatment-emergent anti-PEG antibodies were detected by a direct binding ELISA in 126 out of 268 patients (47%) treated with eflapegrastim-xnst. There was no identified clinically significant effect of anti-drug antibodies on pharmacokinetics, pharmacodynamics, safety or effectiveness of Rolvedon over the treatment duration of 12 weeks. There was no identified clinically significant effect of anti-drug antibodies on the safety profile of Rolvedon during the 12-month follow-up period after the last dose.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term carcinogenicity studies have been performed with eflapegrastim-xnst.
Eflapegrastim-xnst was not mutagenic or clastogenic in a standard battery of genotoxicity tests (bacterial mutagenicity (Ames), Chinese hamster ovary cells chromosomal aberration, rat bone marrow micronucleus).
Eflapegrastim-xnst did not affect reproductive performance or fertility in male or female rats at weekly doses up to 7 times the clinical exposure at the maximum recommended dose of 13.2 mg.
-
14 CLINICAL STUDIES
Patients with Cancer Receiving Myelosuppressive Chemotherapy
The efficacy of Rolvedon to decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anti-cancer drugs was evaluated in two 1:1 randomized, open-label, active-controlled non-inferiority studies of similar design (Study 1 [NCT02643420] and Study 2 [NCT02953340]) that enrolled a total of 643 patients with early-stage breast cancer. Docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 (TC) were administered intravenously every 21 days (on Day 1 of each cycle) for up to 4 cycles. A fixed dose of Rolvedon 13.2 mg/0.6 mL or pegfilgrastim (6 mg/0.6 mL) was administered subcutaneously on Day 2 of each cycle after TC chemotherapy.
The median age of patients enrolled in the two randomized studies was 60 years (Range: 24 to 88), the majority of patients were female (>99%), 77% were White and 12% were Black or African American.
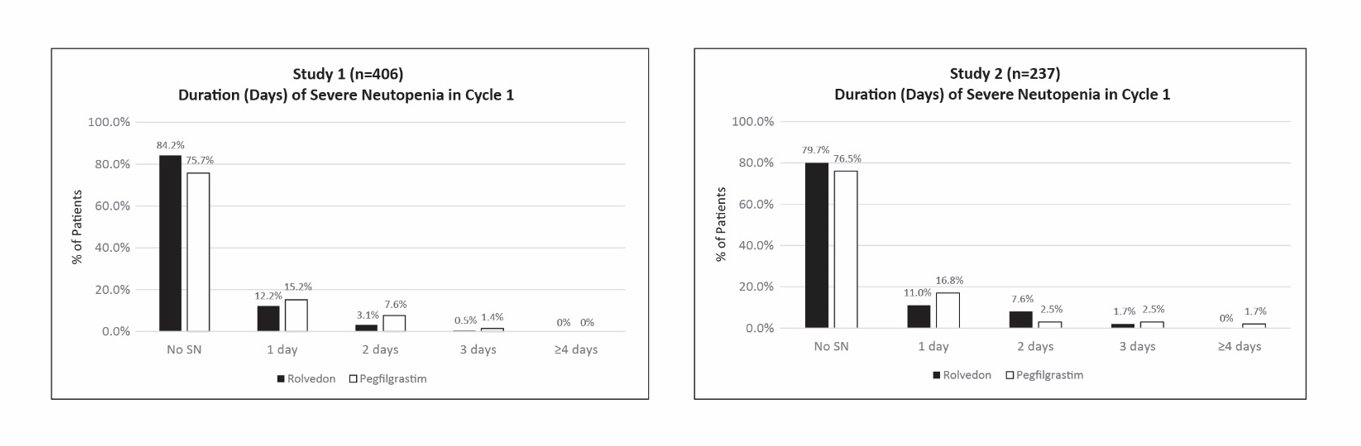
Study 1 enrolled 406 patients; 196 patients to the Rolvedon arm and 210 patients to the pegfilgrastim arm. Study 2 enrolled 237 patients; 118 patients to the Rolvedon arm and 119 patients to the pegfilgrastim arm. Efficacy for both trials was based on the duration of severe neutropenia (DSN) in Cycle 1.
Efficacy results are shown in Table 2. In both studies, Rolvedon was non-inferior to pegfilgrastim. The distributions of the severe neutropenia events in percentage from Cycle 1 for Study 1 and Study 2 are presented in Figure 1.
Table 2. Duration of Severe Neutropenia (DSN) in Cycle 1 (Study 1 and Study 2) aConfidence intervals were obtained using 2.5 percentile and 97.5 percentile of the 100,000 bootstrap samples with treatment as stratification factor. *The non-inferiority of Rolvedon to pegfilgrastim was to be declared if the upper bound of 95% CI of the difference in mean DSN between the treatment arms was <0.62 days. Study 1
Study 2
Rolvedon
(n=196)
Pegfilgrastim
(n=210)
Rolvedon
(n=118)
Pegfilgrastim
(n=119)
Mean DSN (SD) (Days)
0.20 (0.503)
0.35 (0.683)
0.31 (0.688)
0.39 (0.949)
Median DSN (Range) (Days)
0 (0, 3)
0 (0, 3)
0 (0, 3)
0 (0, 7)
Difference in DSN (Days)
-0.148
-0.073
*95% Confidence Intervala
-0.265, -0.033
-0.292, 0.129
Figure 1. Duration of Severe Neutropenia (DSN) by Day in Cycle 1 (Study 1 and Study 2)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Rolvedon (eflapegrastim-xnst) injection is a clear, colorless solution supplied in a single-dose prefilled syringe containing 13.2 mg of eflapegrastim-xnst in 0.6 mL solution, with 29-gauge 1/2 inch pre-attached (staked) needle with a needle guard.
Rolvedon is provided in a dispensing pack containing one sterile 13.2 mg/0.6 mL prefilled syringe (NDC 76961‑101-01).
Store refrigerated at 36°F to 46°F (2°C to 8°C) in the carton to protect from light. Do not shake. Discard syringes stored at room temperature for more than 12 hours. Do not freeze; discard syringe if frozen.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Inform patients to contact their healthcare provider with any questions.
Advise patients of the following risks and potential risks with Rolvedon:
•Splenic rupture and splenomegaly [see Warnings and Precautions (5.1)]
•Acute respiratory distress syndrome [see Warnings and Precautions (5.2)]
•Serious allergic reactions [see Warnings and Precautions (5.3)]
•Sickle cell crisis [see Warnings and Precautions (5.4)]
•Glomerulonephritis [see Warnings and Precautions (5.5)]
•Leukocytosis [see Warnings and Precautions (5.6)]
•Thrombocytopenia [see Warnings and Precautions (5.7)]
•Capillary leak syndrome [see Warnings and Precautions (5.8)]
•Potential for tumor growth stimulatory effects on malignant cells [see Warnings and Precautions (5.9)]
•Increased risk of myelodysplastic syndrome and/or acute myeloid leukemia in patients with breast and lung cancer who receive Rolvedon in conjunction with chemotherapy and/or radiation therapy [see Warnings and Precautions (5.10)]
•Aortitis [see Warnings and Precautions (5.11)]
Instruct patients who self-administer Rolvedon using the single-dose prefilled syringe of the:
•Importance of following the Instructions for Use
•Dangers of reusing syringes
•Importance of following local requirements for proper disposal of used syringes.
Manufactured by:
Spectrum Pharmaceuticals, Inc.
Lake Forest, IL 60045
U.S. License No. 2312 -
PATIENT PACKAGE INSERT
PATIENT INFORMATION
ROLVEDON® (roll veh don)
(eflapegrastim-xnst)
injection
What is Rolvedon?
Rolvedon is a man-made form of granulocyte colony-stimulating factor (G-CSF). G-CSF is a substance produced by the body. It stimulates the growth of neutrophils, a type of white blood cell important in the body’s fight against infection.
It is not known if Rolvedon is safe and effective in children.
Do not take Rolvedon if you have had a serious allergic reaction to eflapegrastim, pegfilgrastim or filgrastim products.
Before receiving Rolvedon, tell your healthcare provider about all of your medical conditions, including if you:
• have a sickle cell disorder
• have kidney problems
• are pregnant or plan to become pregnant. It is not known if Rolvedon can harm your unborn baby. Tell your healthcare provider right away if you become pregnant during treatment with Rolvedon.
• are breastfeeding or plan to breastfeed. It is not known if Rolvedon passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive Rolvedon?
• Rolvedon is given as an injection under your skin (subcutaneous injection) by a healthcare provider. If your healthcare provider decides that the subcutaneous injections can be given at home by you or your caregiver, follow the detailed “Instructions for Use” that comes with your Rolvedon for information on how to prepare and inject a dose of Rolvedon.
• You and your caregiver will be shown how to prepare and inject Rolvedon before you use it.
• You will receive 1 injection of Rolvedon for each cycle of chemotherapy.
• You will receive your injection of Rolvedon about 24 hours after you finish receiving your chemotherapy.
• You should not receive Rolvedon for 14 days before or within 24 hours after your dose of chemotherapy.
• If you miss a dose of Rolvedon, talk to your healthcare provider about when you should receive your next dose.
What are the possible side effects of Rolvedon?
Rolvedon may cause serious side effects, including:
• Spleen rupture. Your spleen may become enlarged and can rupture. A ruptured spleen can cause death. Call your healthcare provider right away if you have pain in the left upper stomach-area or your left shoulder.
• A serious lung problem called Acute Respiratory Distress Syndrome (ARDS). Call your healthcare provider or get emergency help right away if you have shortness of breath with or without a fever, trouble breathing, or a fast rate of breathing.
• Serious allergic reactions. Rolvedon can cause serious allergic reactions. These reactions can cause a rash all over your whole body, shortness of breath, wheezing, dizziness, swelling around your mouth or eyes, fast heart rate, and sweating. If you have any of these symptoms, stop using Rolvedon and call your healthcare provider or get emergency medical help right away.
• Sickle cell crises. You may have a serious sickle cell crisis, which could lead to death, if you have a sickle cell disorder and receive Rolvedon. Call your healthcare provider right away if you develop symptoms of sickle cell crisis such as pain or difficulty breathing.
• Kidney injury (glomerulonephritis). Rolvedon can cause kidney injury. Call your healthcare provider right away if you develop any of the following symptoms:
o swelling of your face or ankles
o blood in your urine or dark colored urine
o you urinate less than usual
• Increased white blood cell count (leukocytosis). Your healthcare provider will check your blood count during treatment with Rolvedon.
• Decreased platelet count (thrombocytopenia). Your healthcare provider will check your blood during treatment with Rolvedon. Tell your healthcare provider if you have unusual bleeding or bruising during treatment with Rolvedon. This could be a sign of decreased platelet counts, which may reduce the ability of your blood to clot.
• Capillary Leak Syndrome. Rolvedon can cause fluid to leak from blood vessels into your body’s tissues. This condition is called “Capillary Leak Syndrome” (CLS). CLS can quickly cause you to have symptoms that may become life-threatening. Get emergency help right away if you develop any of the following symptoms:
o swelling or puffiness and are urinating less than usual
o trouble breathing
o swelling of your stomach area (abdomen) and feeling of fullness
o dizziness or feeling faint
o a general feeling of tiredness
• Myelodysplastic syndrome and acute myeloid leukemia. If you have breast cancer or lung cancer, when Rolvedon is used with chemotherapy and radiation therapy, or with radiation therapy alone, you may have an increased risk of developing a precancerous blood condition called myelodysplastic syndrome (MDS) or a blood cancer called acute myeloid leukemia (AML). Symptoms of MDS and AML may include tiredness, fever, and easy bruising or bleeding. Call your healthcare provider if you develop these symptoms during treatment with Rolvedon.
• Inflammation of the aorta (aortitis). Inflammation of the aorta (the large blood vessel which transports blood from the heart to the body) has been reported in patients who received pegfilgrastim products. Symptoms may include fever, abdominal pain, feeling tired, and back pain. Call your healthcare provider if you experience these symptoms.
The most common side effects of Rolvedon include:
• tiredness
• nausea
• diarrhea
• bone pain
• headache
• fever
• decreased red blood cell count
• rash
• muscle and joint pain
• back pain
These are not all the possible side effects of Rolvedon.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Rolvedon?
• Store Rolvedon in the refrigerator between 36°F to 46°F (2°C to 8°C).
• Do not freeze Rolvedon. Throw away (dispose of) any Rolvedon that has been frozen.
• Store Rolvedon in the original carton to protect from light.
• Do not shake Rolvedon.
• Take the carton out of the refrigerator and place the sealed blister tray on a clean flat surface for at least 30 minutes to allow it to reach room temperature before use.
• Throw away (dispose of) any Rolvedon that has been left at room temperature, 68ºF to 77ºF (20ºC to 25ºC), for more than 12 hours.
Keep Rolvedon and all medicines out of the reach of children.
General information about the safe and effective use of Rolvedon.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Rolvedon for a condition for which it was not prescribed. Do not give Rolvedon to other people, even if they have the same symptom that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Rolvedon that is written for health professionals.
What are the ingredients in Rolvedon?
Active ingredient: eflapegrastim-xnst
Inactive ingredients: citric acid monohydrate, mannitol, polysorbate 80, and sodium chloride in Water for Injection. Sodium hydroxide may be used to adjust pH to 5.5 during manufacturing.
Manufactured by: Spectrum Pharmaceuticals, Inc. Lake Forest, IL 60045
U.S. License No. 2312
For more information, go to www.rolvedon.com or call 1-888-713-0688.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 11/2023
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
ROLVEDON® [roll veh don]
(eflapegrastim-xnst)
injection, for subcutaneous use
Single-Dose Prefilled Syringe
This Instructions for Use contains information on how to inject Rolvedon. Read this Instructions for Use before using Rolvedon and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Important Information You Need To Know Before Injecting Rolvedon
• It is important that you do not try to give the injection unless you or your caregiver have received training from your healthcare provider.
• Rolvedon is for use under the skin (subcutaneous injection) only.
• To help reduce needle stick injuries, each prefilled syringe has a needle guard that is automatically activated to cover the needle after the injection is complete.
• Make sure the name Rolvedon appears on the carton and prefilled syringe label.
• Do not use a Rolvedon prefilled syringe after the expiration date on the label.
• Do not use the Rolvedon prefilled syringe if it has been dropped on a hard surface. The prefilled syringe may be broken even if you cannot see the break. Use a new prefilled syringe.
• Do not use the Rolvedon prefilled syringe if the seal on the carton or the seal on the blister tray is broken.
• Do not shake the Rolvedon prefilled syringe.
• Do not touch the needle guard clips before use. Touching them may cause the needle guard to be activated too early.
• Do not remove the needle cap until just before you give the injection.
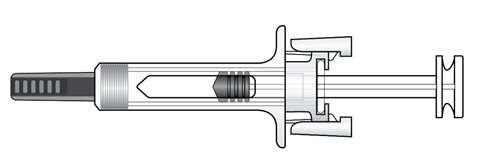
Rolvedon prefilled syringe parts
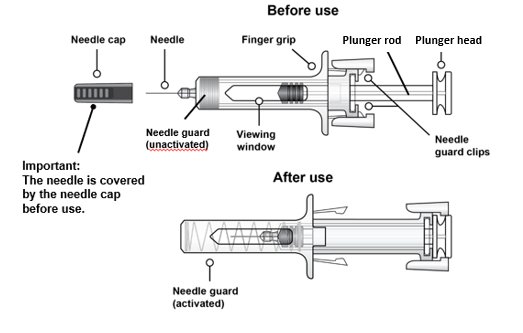
Figure A
Storing the Rolvedon prefilled syringe
• Store Rolvedon in the refrigerator between 36°F to 46°F (2°C to 8°C).
• Do not freeze Rolvedon. Throw away (dispose of) any Rolvedon that has been frozen.
• Store Rolvedon in the original carton to protect from light.
• Take the carton out of the refrigerator and place the sealed blister tray on a clean flat surface for at least 30 minutes to allow it to reach room temperature before use.
• Throw away (dispose of) any Rolvedon that has been left at room temperature, 68ºF to 77ºF (20ºC to 25ºC), for more than 12 hours.
Keep Rolvedon and all medicines out of the reach of children.
Preparing to inject Rolvedon
Step 1. Remove the carton from refrigerator
• Remove the carton from the refrigerator (see Figure B).
• Check the expiration date on the carton (see Figure C).
Do not use the prefilled syringe if it is expired or if the carton has been previously opened.
• Open the carton and remove the blister tray.
Figure B
Figure C
Step 2. Allow Rolvedon to reach room temperature
Place the sealed blister tray on a clean flat surface and wait 30 minutes to reach room temperature (see Figure D). Do not warm up the prefilled syringe in any other way.

Figure D
Step 3. Gather the supplies needed for your injection
Gather the following supplies for the injection (see Figure E):
• 1 sterile cotton ball or gauze pad
• 1 alcohol wipe
• 1 sharps disposal container. See “Disposing of used Rolvedon prefilled syringes” at the end of this Instructions for Use.

Figure EStep 4. Wash your hands
Wash your hands with soap and water.
Step 5. Remove the prefilled syringe from the blister tray
• Open the tray by peeling away the tray cover.
• To remove the prefilled syringe, grip the prefilled syringe by the center of the viewing window as shown in Figure F.
• Do not remove the prefilled syringe by the plunger rod, plunger head, or the needle cap.
• Do not touch the needle guard clips, as it could activate the needle guard.
• Do not remove the needle cap until you are ready to inject.
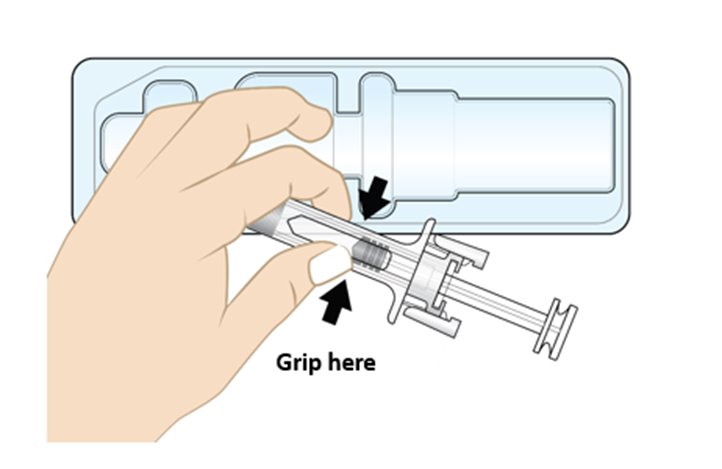
Figure F
Step 6. Inspect the prefilled syringe and medicine
• Visually inspect the prefilled syringe. Do not use the prefilled syringe if it appears damaged (for example, if the syringe is cracked or broken, or if the plunger rod is detached or broken) or if the needle cap is missing.
• Check the expiration date on the prefilled syringe label. Do not use if the expiration date has passed.
• Check the medicine inside the prefilled syringe through the viewing window (see Figure G). The medicine inside should be clear and colorless. It is normal to see small air bubbles in the medicine. It does not need to be removed.
• Do not use the prefilled syringe if:
o the medicine appears discolored or cloudy.
o the medicine contains lumps, flakes, or particles.
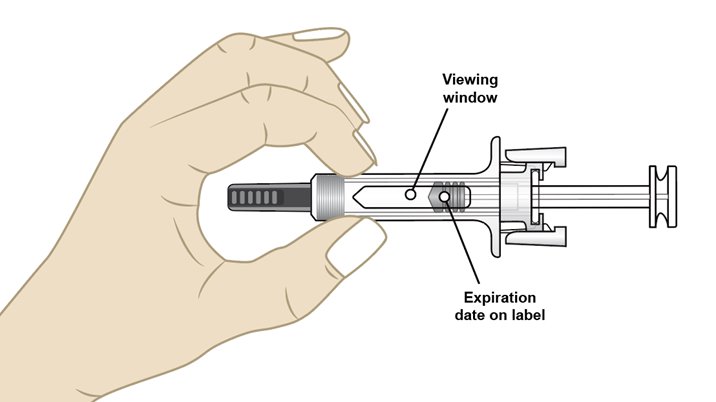
Figure GStep 7. Select the injection site
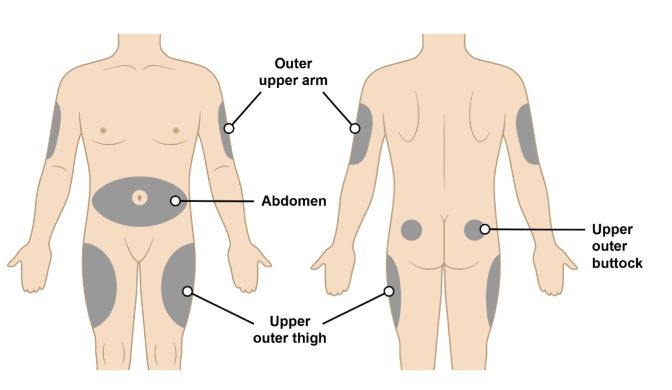
Figure H•The recommended injection sites for a subcutaneous injection are the (see Figure H):
o Abdomen (except for a two-inch area surrounding the navel)
o Upper outer thighoOuter upper arm (only if someone else is giving you the injection)
o Upper outer buttock (only if someone else is giving you the injection)
• Do not inject into area of the skin that is tender, damaged, bruised, scarred or hard.
• Do not inject into moles, scars, birthmarks or stretchmarks.
• If you want to use the same injection site, make sure it is not the same spot on the injection site you used for a previous injection.
Step 8. Clean the injection site
• Clean the injection site with an alcohol wipe (see Figure I). • Allow the area of skin to dry before injecting.
Do not touch or blow on the cleaned area before injecting.
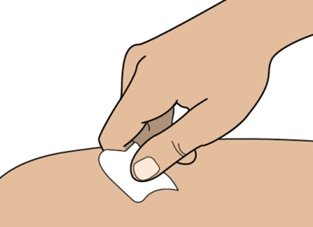
Figure IInjecting Rolvedon
Step 9. Remove needle cap
• Do not remove the needle cap until you are ready to inject. When ready for the injection, carefully remove the needle cap by pulling it straight off (see Figure J).
• When removing the needle cap, hold the prefilled syringe as shown in Figure J.
• Throw away (dispose of) the needle cap in the sharps disposal container.
• Do not put the needle cap back onto the prefilled syringe after it has been removed. This can damage the needle and cause needle stick injuries.
• Do not hold the prefilled syringe by the plunger rod or touch the needle guard clips when removing the needle cap.
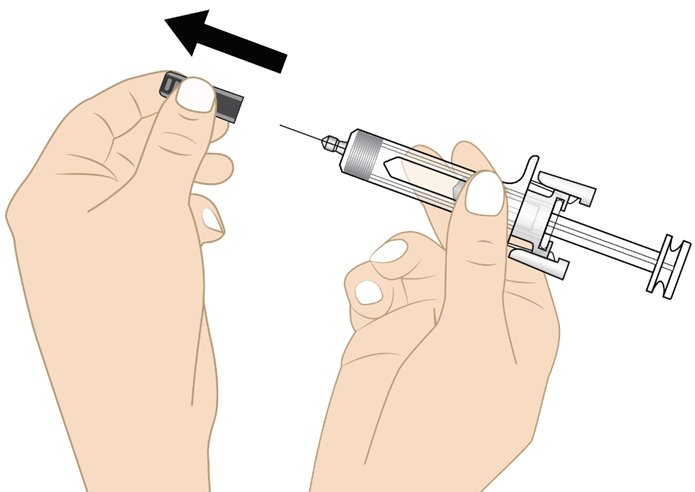
Figure JStep 10. Insert the needle
• Hold the prefilled syringe at a 45 to 90-degree angle (see Figure K).
• Using your other hand, gently pinch the cleaned injection site as shown in Figure K.
• Fully insert the needle into the skin in a quick “dart-like” motion.
Do not touch the plunger head while inserting the needle into the skin.
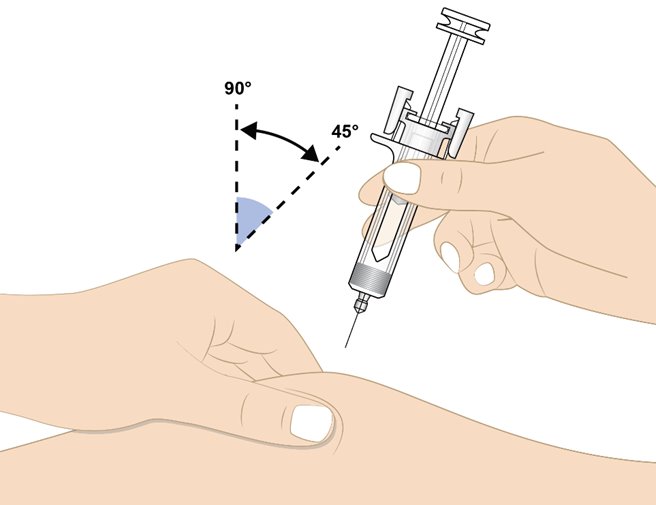
Figure K
Step 11. Inject Rolvedon
• Release the pinched skin.
• Hold the prefilled syringe as shown in Figure L with your thumb on the plunger head ready to inject.
• Slowly press the plunger head down to inject the medicine.
• Hold the syringe steadily in place while the needle is inserted.
Note: You may feel some resistance, this is normal.
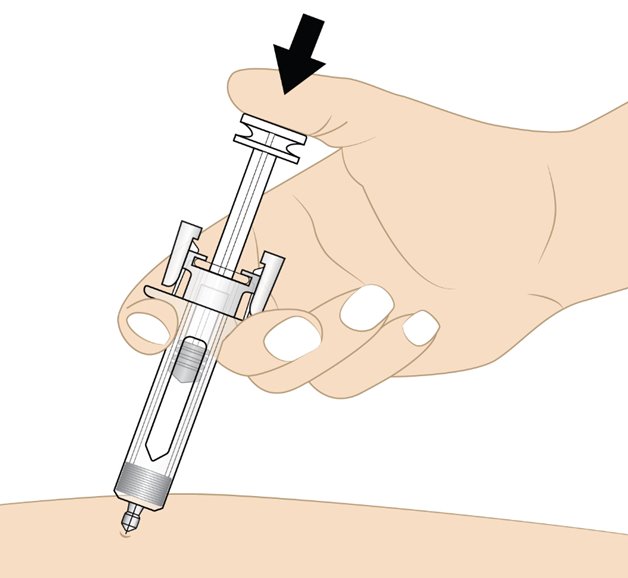
Figure L
• Push the plunger head down until it reaches the bottom to make sure all of the medicine is injected (see Figure M).
• You may hear a click when the plunger head reaches the bottom.
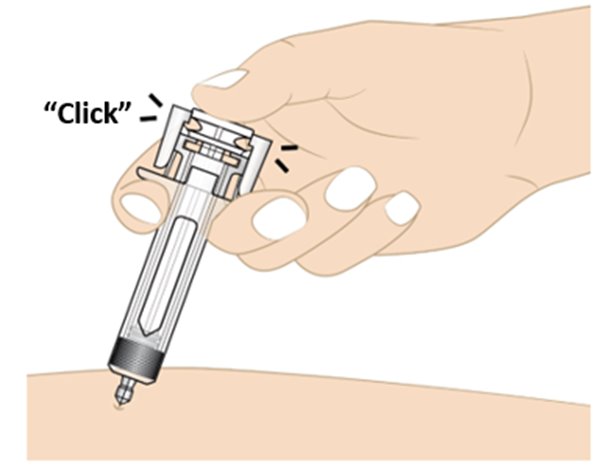
Figure MStep 12. Activate the needle guard
• Gently lift your thumb to release the plunger until the entire needle is covered by the needle guard. Then remove the prefilled syringe from the injection site at the same angle as inserted as shown in Figure N.• If there is bleeding, lightly press a cotton ball or gauze on the injection site. 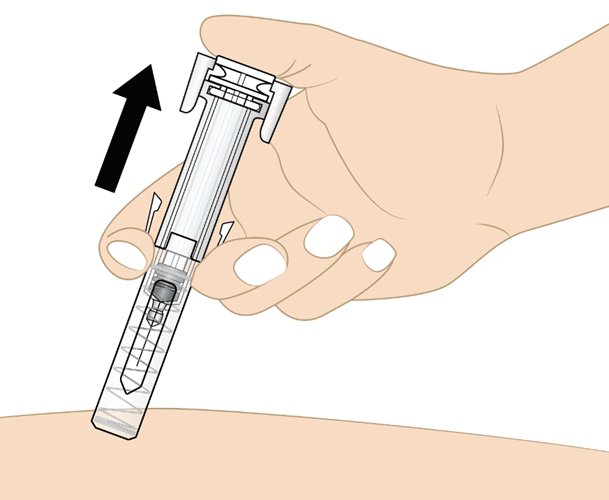
Figure NDisposing of used Rolvedon prefilled syringes
• Put the used prefilled syringe and needle cap in an FDA-cleared sharps disposal container right away after use (see Figure O). Do not throw away (dispose of) the prefilled syringe in your household trash.
• If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
o made of a heavy-duty plastic,
o can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
o upright and stable during use,
o leak-resistant, and
o properly labeled to warn of hazardous waste inside the container.
• When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
• Do not reuse the prefilled syringes or throw them into household waste.
• Do not recycle prefilled syringes or sharps disposal container.
• Do notattempt to remove the needle from the prefilled syringe.
Important: Keep the sharps disposal container out of the reach of children.
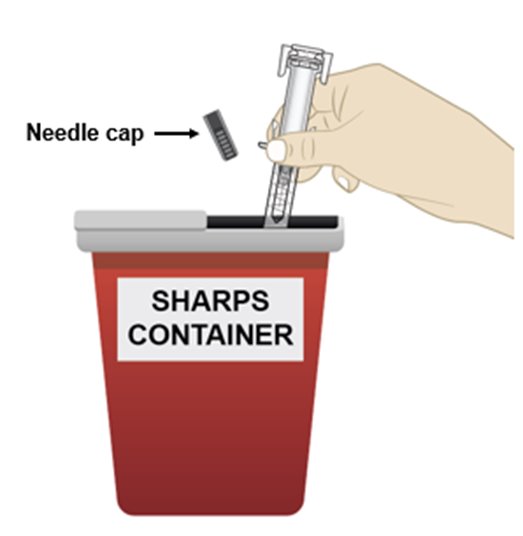
Figure O
Manufactured by: Spectrum Pharmaceuticals, Inc., Lake Forest, IL 60045
U.S. License No. 2312
For more information, go to www.rolvedon.com or call 1-888-713-0688.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 11/2023
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Rolvedon
(eflapegrastim-xnst)
injection
13.2 mg/0.6 mL
Rolvedon
(eflapegrastim-xnst)
injection
13.2 mg/0.6 mL
For subcutaneous injection
Sterile Solution – No Preservative
One 0.6 mL Single-dose Pre-filled Syringe
Rolvedon
(eflapegrastim-xnst)
injection
13.2 mg/0.6 mL
NDC 76961-101-01
Rx only
For subcutaneous injection
Sterile Solution – No Preservative
One 0.6 mL Single-dose Pre-filled Syringe
-
INGREDIENTS AND APPEARANCE
ROLVEDON
eflapegrastim-xnst injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76961-101 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EFLAPEGRASTIM (UNII: UT99UG9QJX) (EFLAPEGRASTIM - UNII:UT99UG9QJX) EFLAPEGRASTIM 13.2 mg in 0.6 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 2.52 mg in 0.6 mL MANNITOL (UNII: 3OWL53L36A) 30 mg in 0.6 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.72 mg in 0.6 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 5.26 mg in 0.6 mL WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76961-101-01 0.6 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 10/18/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761148 10/18/2022 Labeler - Spectrum Pharmaceuticals, Inc. (790888002)